Acid Strength Chart
Acid Strength Chart - What are strong and weak acids? Web in this article, we will talk about acid strength and the factors which affect it. Acid strength is the measure of the ability of the acid to lose its h + ion. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. Web measures of acid strength. Acids or bases with weak bonds easily dissociate into ions and are called strong acids or bases. Each acid and each base has an associated ionization constant that corresponds to its acid or base strength. Summary list of characteristics for strong and weak acids and bases. Acids or bases with strong bonds exist predominately as molecules in solutions and are called weak acids or bases. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. The stronger an acid is, the more easily it loses a proton,. Summary list of characteristics for strong and weak acids and bases. Web in this article, we will talk about acid strength and the factors which affect it. Web measures of acid strength. Each acid and each base has an associated ionization constant that corresponds to its acid or. Web bond strength principle. Web in this article, we will talk about acid strength and the factors which affect it. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Each acid and each base has an associated ionization constant that corresponds to its acid or. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Web bond strength principle. Acid strength is the measure of the ability of the acid to lose its h + ion.. Each acid and each base has an associated ionization constant that corresponds to its acid or base strength. The stronger an acid is, the more easily it loses a proton,. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. Web measures of acid strength. Acid strength is the measure of the ability of the. Web measures of acid strength. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. Acid strength is the measure of the ability of the acid to lose its h + ion. The stronger an acid is, the more easily it loses a proton,. Web in this article, we will talk about acid strength and. Acid strength is the measure of the ability of the acid to lose its h + ion. The stronger an acid is, the more easily it loses a proton,. Summary list of characteristics for strong and weak acids and bases. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. Acids or bases with weak bonds easily dissociate into ions and are called strong acids or bases. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Acid strength is the. Web measures of acid strength. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. The stronger an acid is, the more easily it loses a proton,. Each acid and each. Summary list of characteristics for strong and weak acids and bases. Acid strength is the measure of the ability of the acid to lose its h + ion. Stronger acids have a larger and a smaller logarithmic constant ( ) than weaker acids. The usual measure of the strength of an acid is its acid dissociation constant ( ), which. Web in this article, we will talk about acid strength and the factors which affect it. Acids or bases with strong bonds exist predominately as molecules in solutions and are called weak acids or bases. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Each. What are strong and weak acids? Summary list of characteristics for strong and weak acids and bases. Web measures of acid strength. Acid strength is the measure of the ability of the acid to lose its h + ion. Web bond strength principle. Each acid and each base has an associated ionization constant that corresponds to its acid or base strength. Acids or bases with strong bonds exist predominately as molecules in solutions and are called weak acids or bases. The usual measure of the strength of an acid is its acid dissociation constant ( ), which can be determined experimentally by titration methods. Acids or bases with weak bonds easily dissociate into ions and are called strong acids or bases.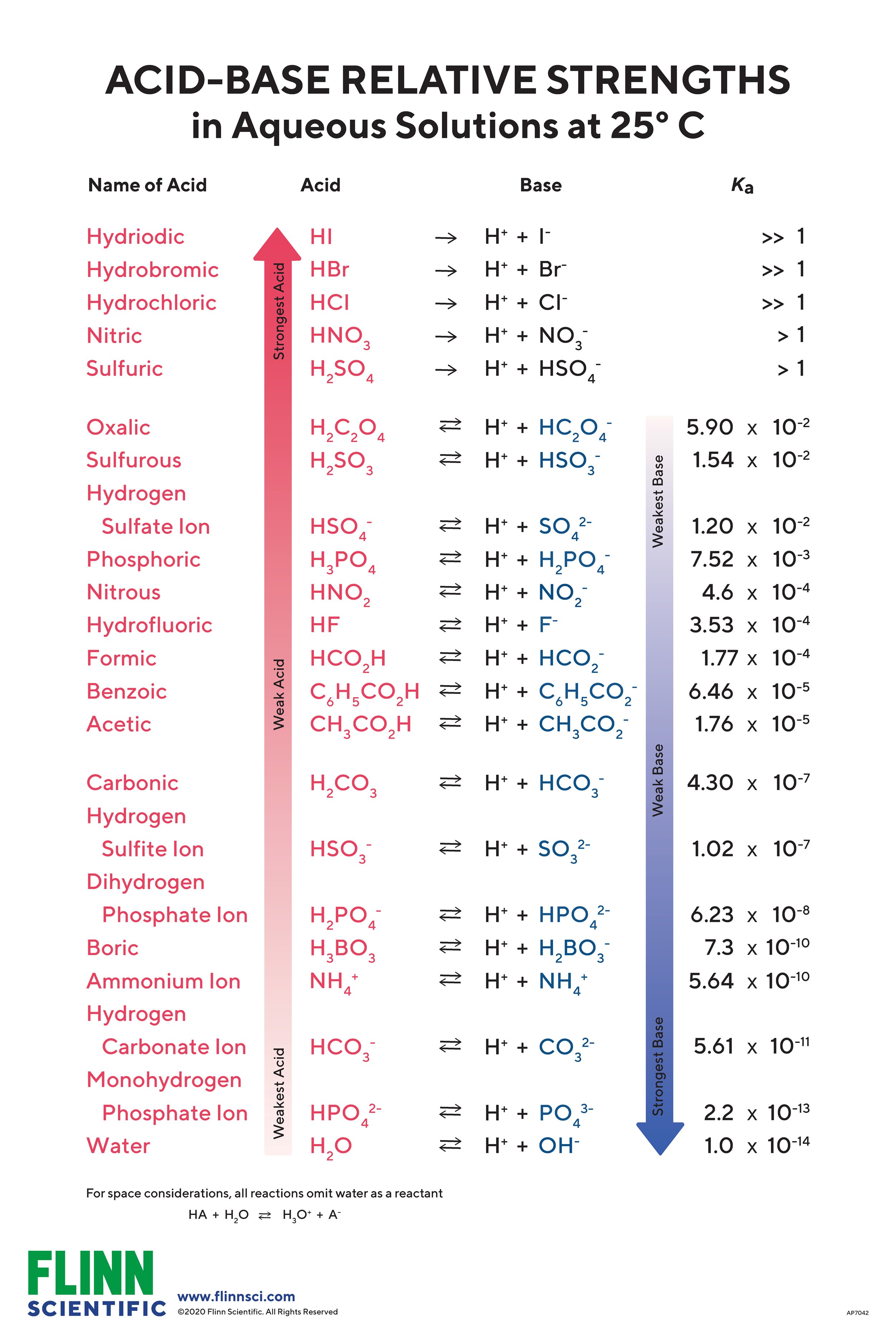
AcidBase Strength Chart
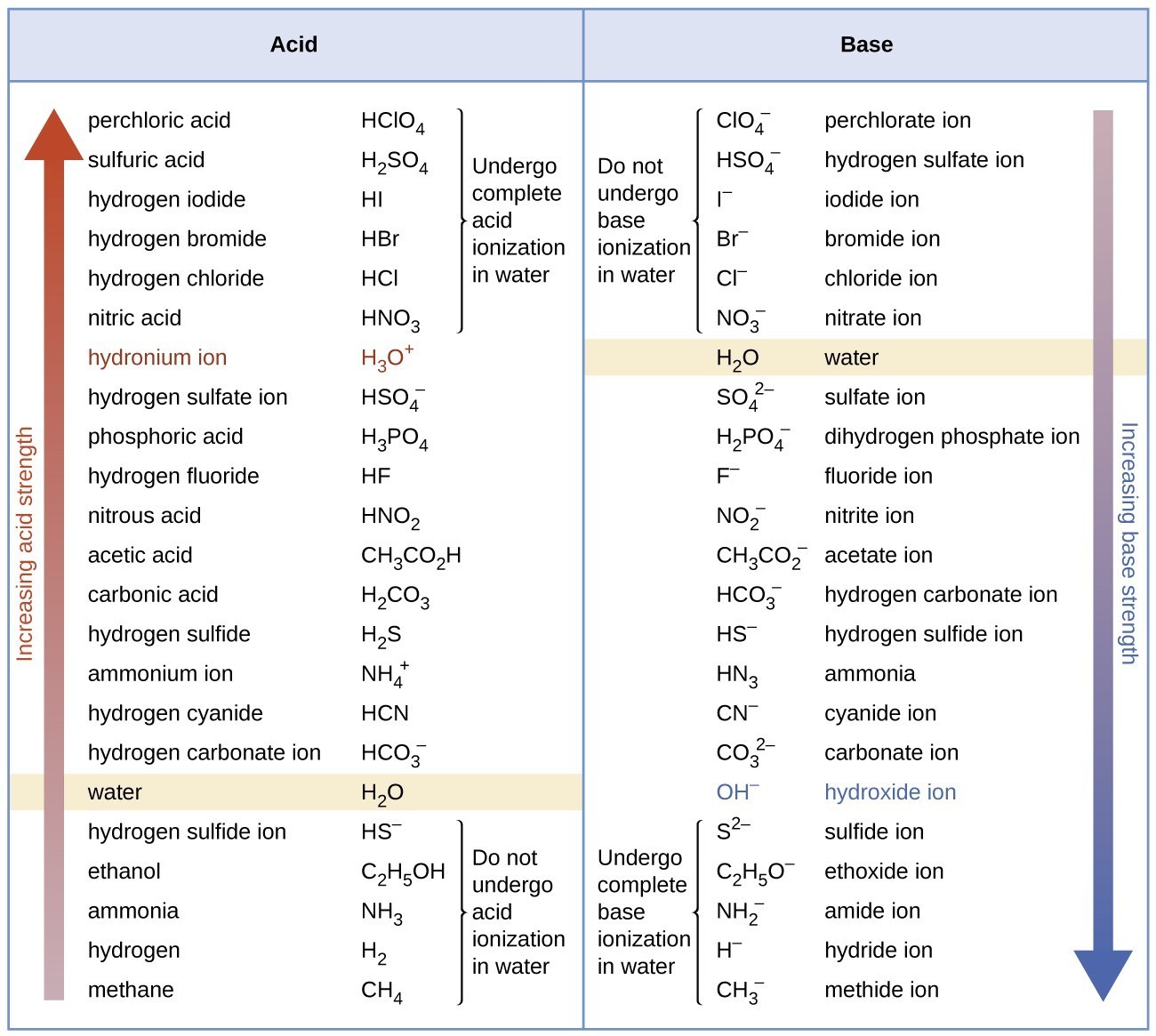
Relative Strengths of Acids and Bases Chemistry
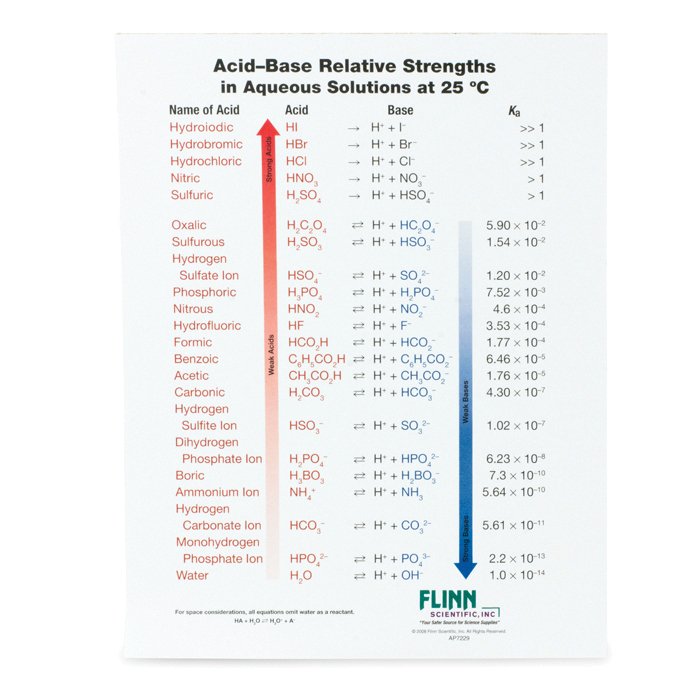
AcidBase Strength Charts for Chemistry
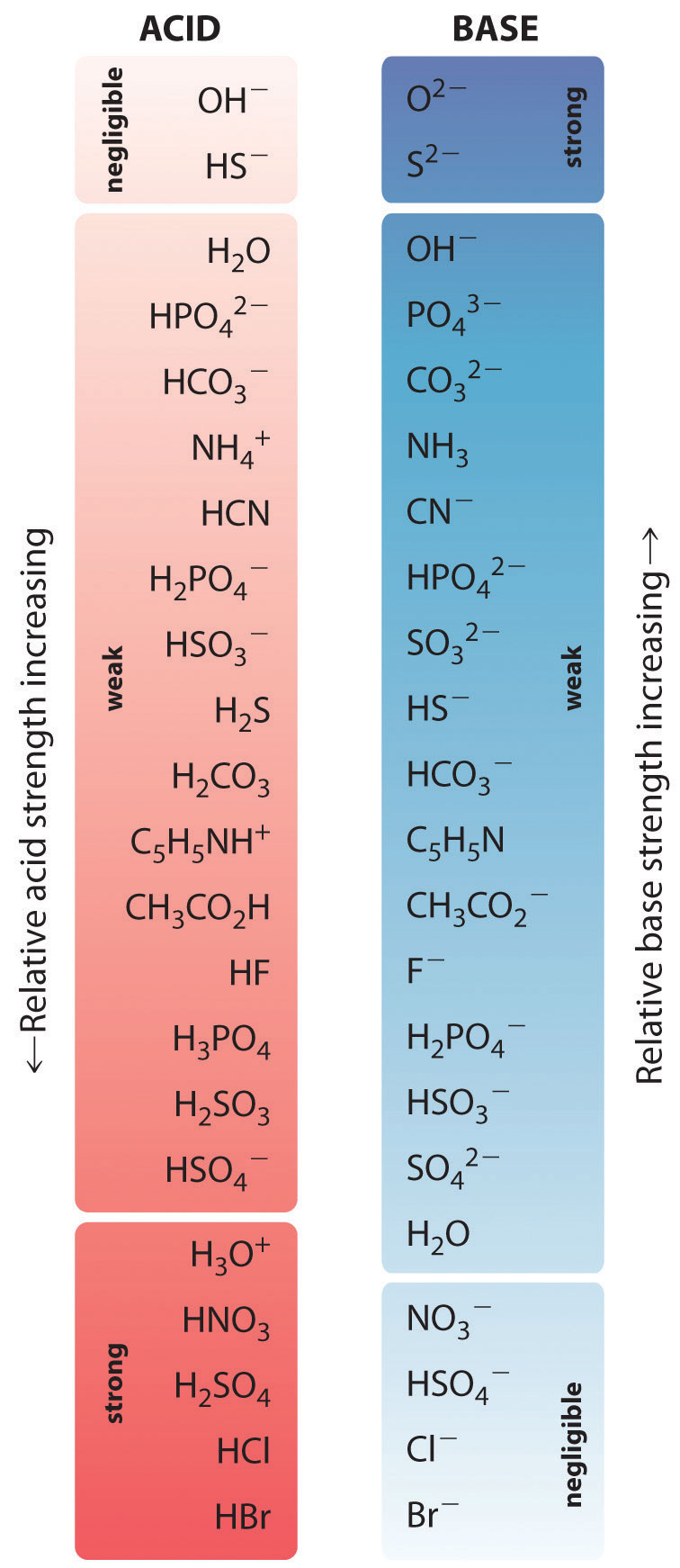
Acid Strengths Table
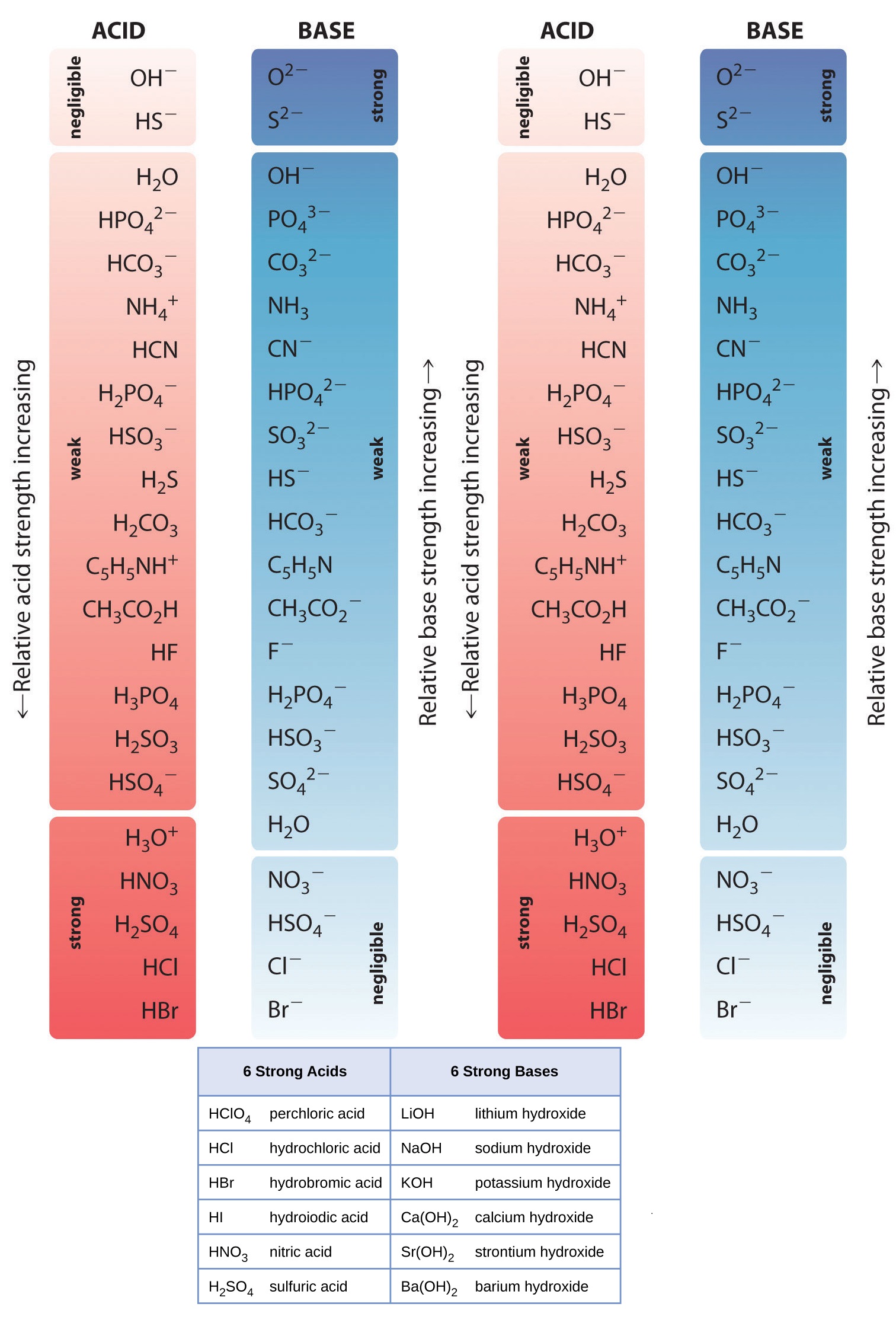
List of Strong Acids & Bases in Order StudyPK

A Guide to Acids, Acid Strength, and Concentration Compound Interest
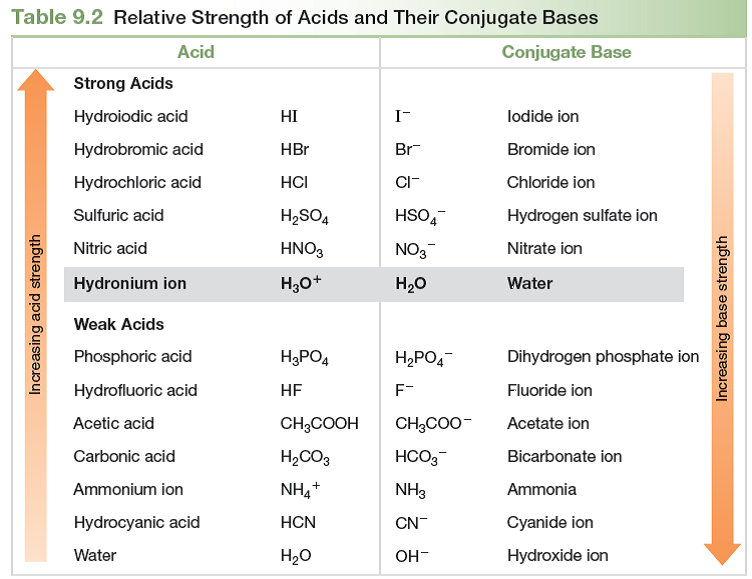
Solved Acid and Base Strength Use the data in Tables 9.2 and 9
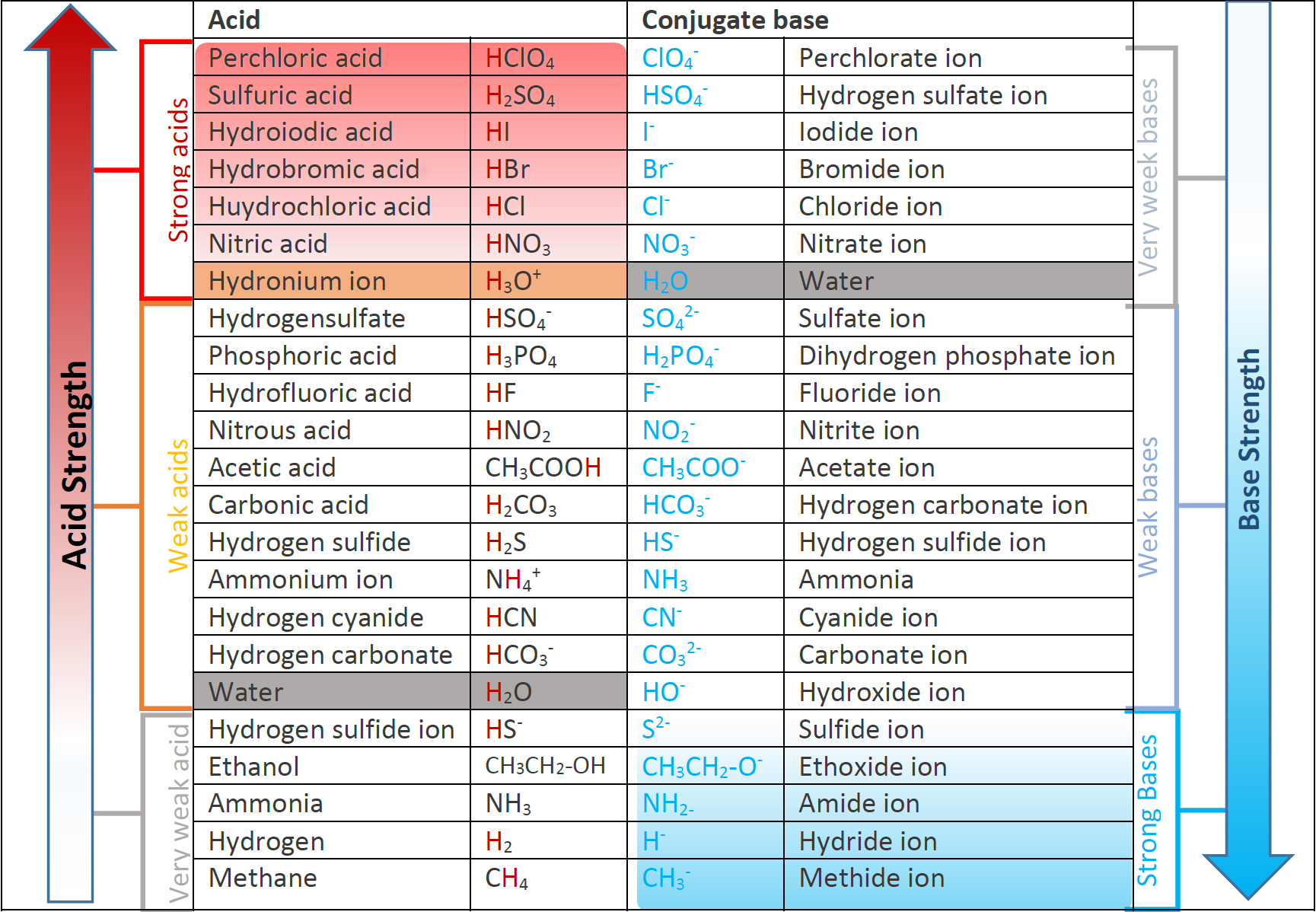
6.3 Strength of acids and bases Chemistry LibreTexts
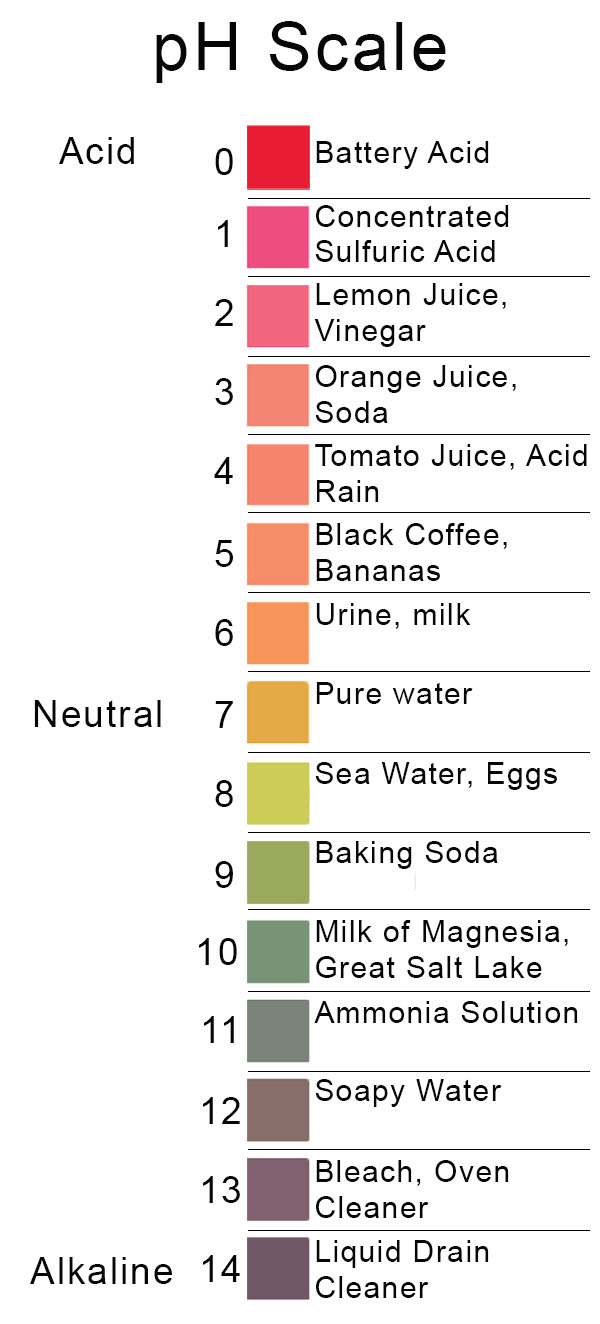
Back to Basics Acids, Bases & the pH Scale Precision Laboratories
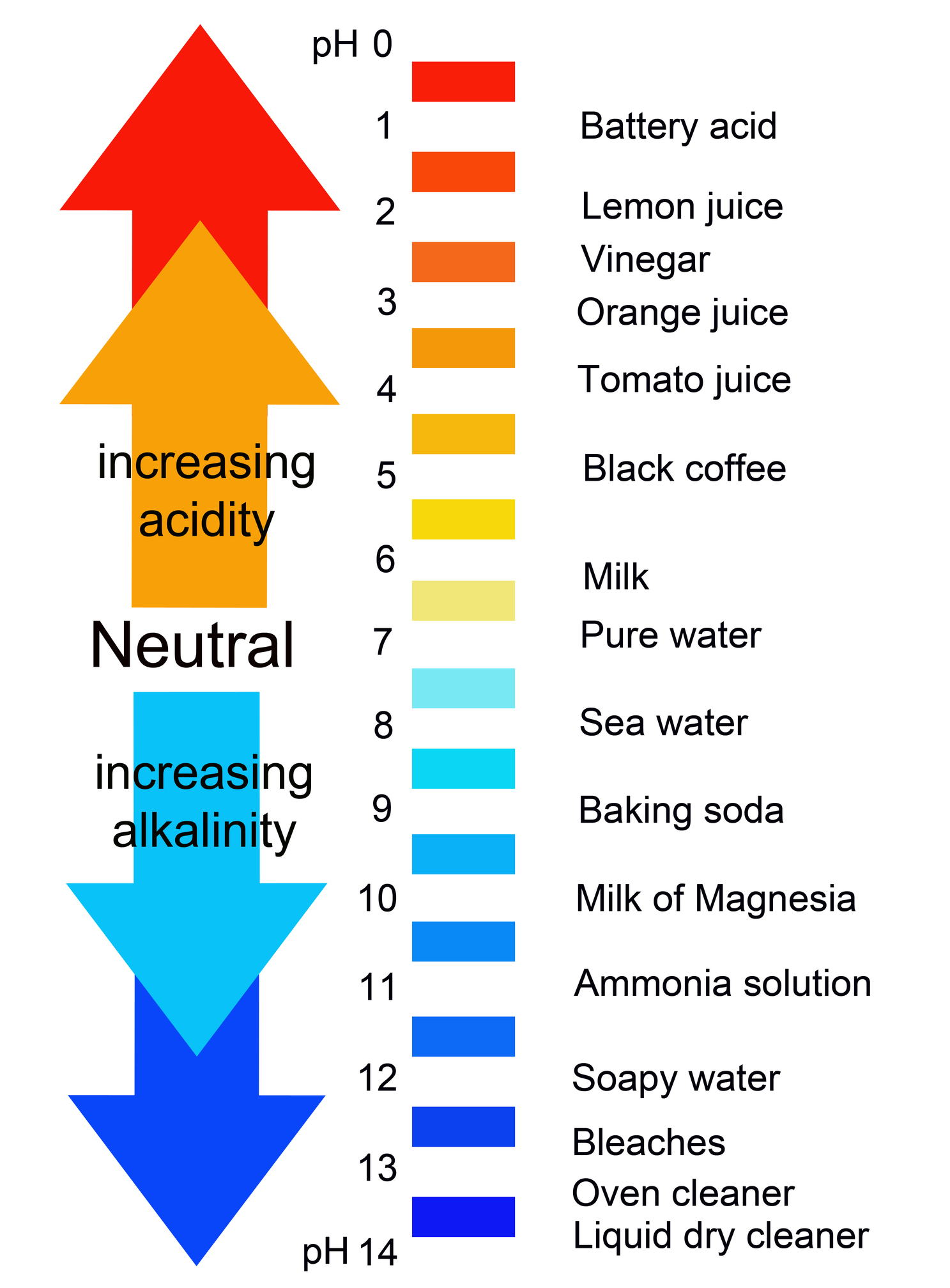
Acids and Bases
Web In This Article, We Will Talk About Acid Strength And The Factors Which Affect It.
The Stronger An Acid Is, The More Easily It Loses A Proton,.
Stronger Acids Have A Larger And A Smaller Logarithmic Constant ( ) Than Weaker Acids.
Related Post: