Activity Chart Chemistry
Activity Chart Chemistry - It shows you how to tell if a single replacement reaction will work or if there will be no. They are called active metals. Web the activity series is a list of elements in decreasing order of their reactivity. The activity series of metals. Specifically, use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction. Define activity and determine which element in a reaction is more active given the balanced chemical equation. Web in chemical thermodynamics, activity (symbol a) is a measure of the effective concentration of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Zncl 2 + mg mgcl 2 magnesium is above zinc so the reaction happens zncl 2 + cu no reaction copper is below zinc so no reaction. An activity series is a list of metals arranged in order of decreasing ease of oxidation. Web the activity series is a list of elements in decreasing order of their reactivity. Web this chemistry video tutorial explains the activity series of metals and elements such as hydrogen. Web writing equations to make an activity series. Web the activity series is a list of elements in decreasing order of their reactivity. Mg (s) + 2 h +. Web the activity series is a list of elements in decreasing order of their reactivity. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: An activity series is a list of metals arranged in order of decreasing ease of oxidation. The primary difference between metals is the ease. Web activity series of metals; Mg (s)+cucl2(aq) → mgcl2(aq) + cu (s) answer. Specifically, use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction. Web the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals,. Define activity and determine which element in a reaction is more active given the balanced chemical equation. How do you use this series? Most active or most easily oxidized: Web writing equations to make an activity series. The activity series of metals. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Most active or most easily oxidized: They are called active metals. An activity series is a list of metals arranged in order of decreasing ease of oxidation. Web the activity series is a list of elements in decreasing order of their reactivity. Lithium, sodium, and potassium all react with water, for example. Most active or most easily oxidized: A metal listed above another metal will replace that metal in a single replacement (single displacement) reaction. Web the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each. The activity series of metals. 613 views 3 years ago chemistry. Web the activity series is a list of elements in decreasing order of their reactivity. Web in chemical thermodynamics, activity (symbol a) is a measure of the effective concentration of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a. Examples of predicting products with an activity series chart. Activity series of metals and reaction prediction. Web define activity and determine which element in a reaction is more active given the balanced chemical equation; The activity series of metals. Web the activity series is a chart of metals listed in order of declining relative reactivity. The reaction does not occur in this situation. Web the activity series is a chart of metals listed in order of declining relative reactivity. Specifically, use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a. Web the activity series is a list of elements in decreasing order of their reactivity. Write an activity series for 3 or more elements based on a multiple replacement reactions. Does the following reaction occur? How do you use this series? Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) The reaction does not occur in this situation. The table below is an activity series of. 613 views 3 years ago chemistry. Web define activity and determine which element in a reaction is more active given the balanced chemical equation; An activity series is a list of metals arranged in order of decreasing ease of oxidation. The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. Web the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Predicting products of chemical reactions. In today's video, we are looking at how to use an activity series chart for metals and nonmetals. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Web the activity series is a list of elements in decreasing order of their reactivity. Web the activity of metals. Zncl 2 + mg → mgcl 2 magnesium is above zinc so the reaction happens zncl 2 + cu → no reaction copper is below zinc so no. It shows you how to tell if a single replacement reaction will work or if there will be no. The reaction does not occur in this situation.
Periodic Table of The Elements Educational Science Chart Chemistry
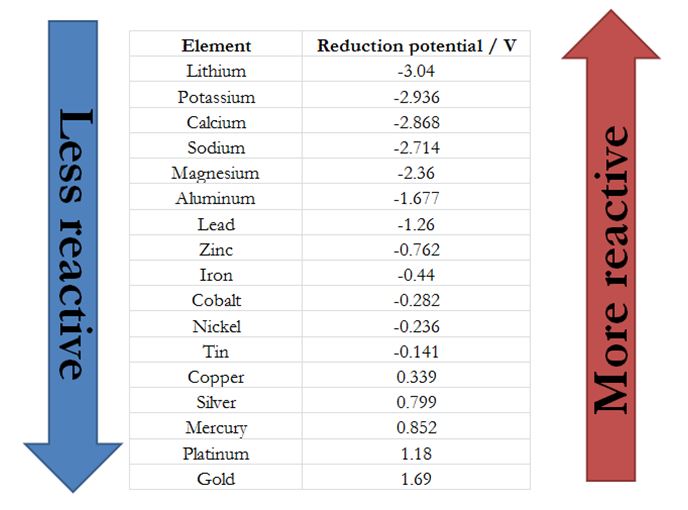
Using Chemistry to Find that Silver Lining Chemical Education Xchange

Pin by redacted on Chemistry Education Chemistry lessons, Chemistry
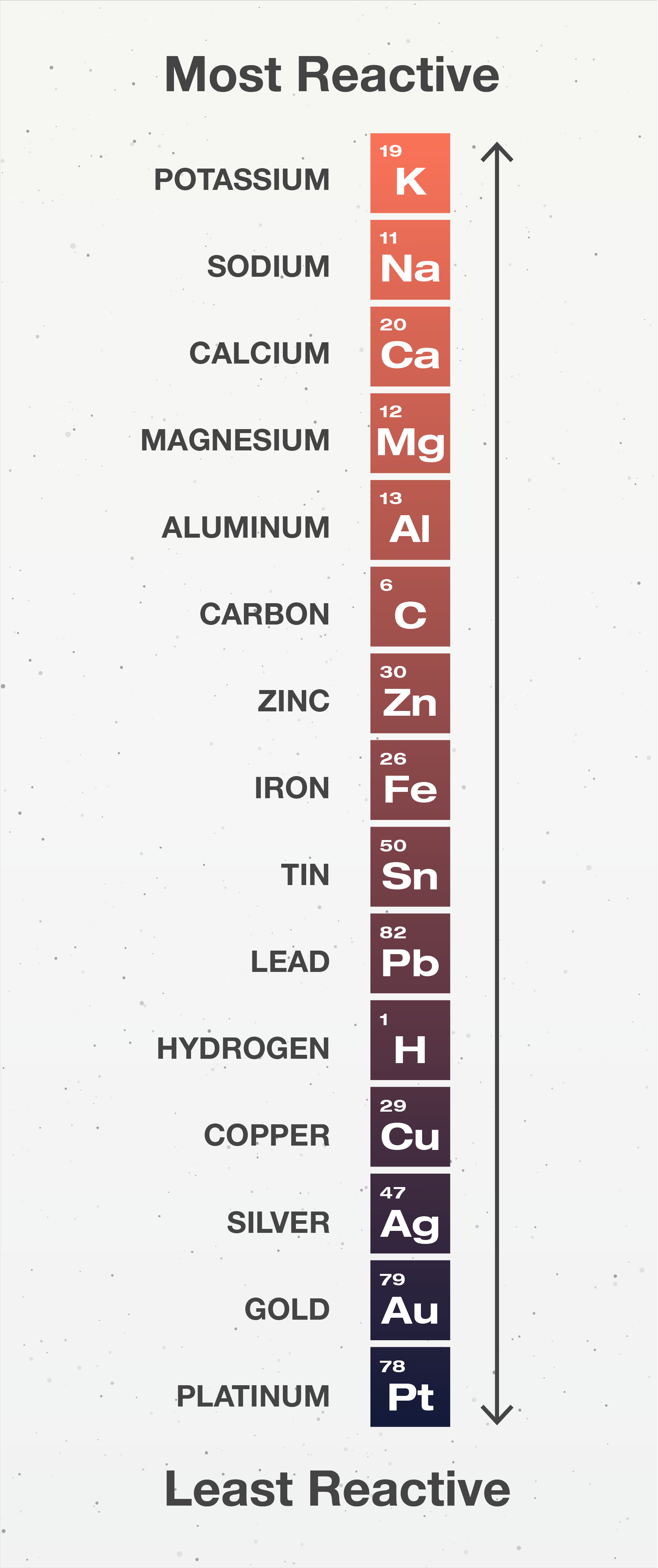
5.1 Activity Series Chemistry LibreTexts
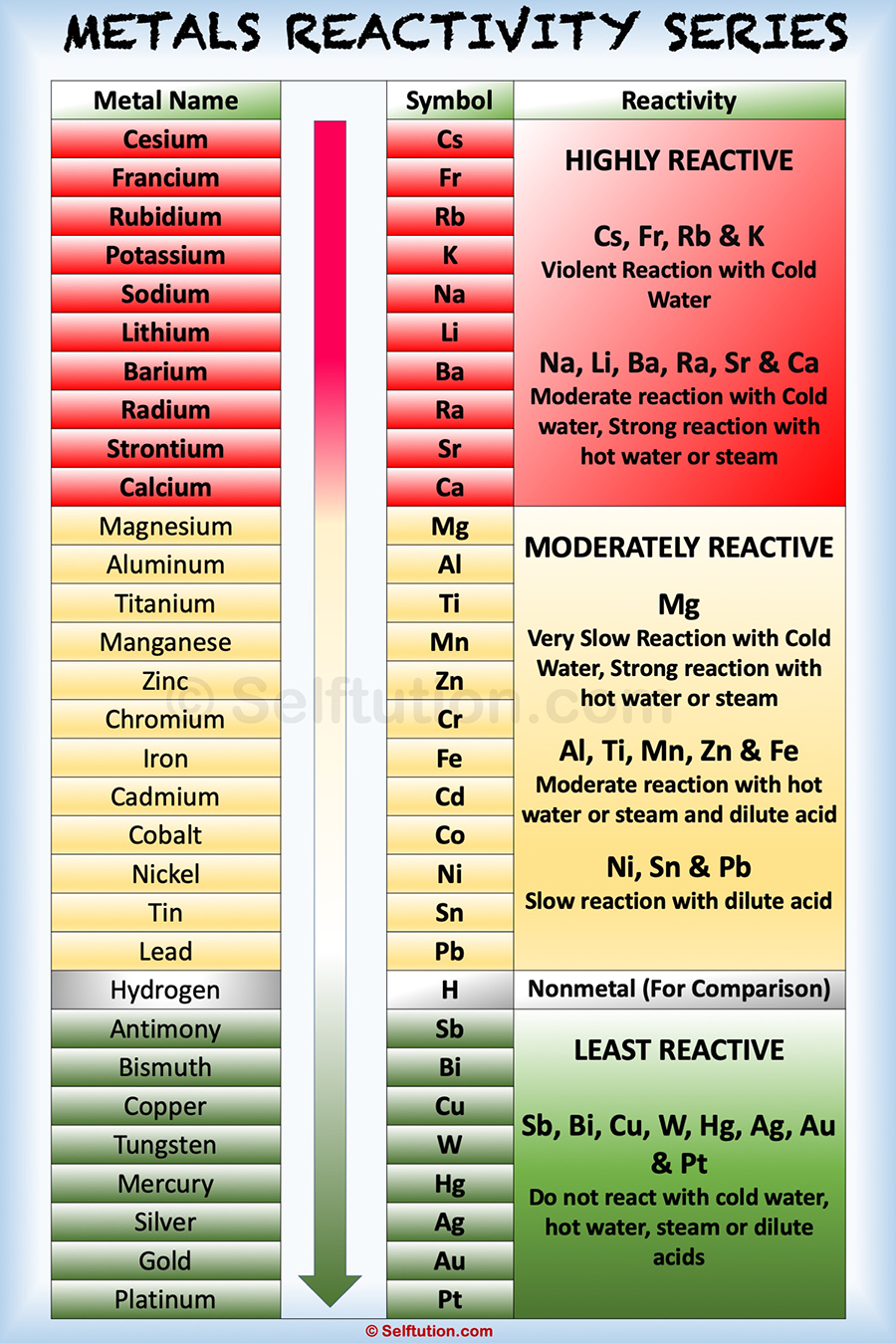
Reactivity Series of Metals and Nonmetals » Selftution
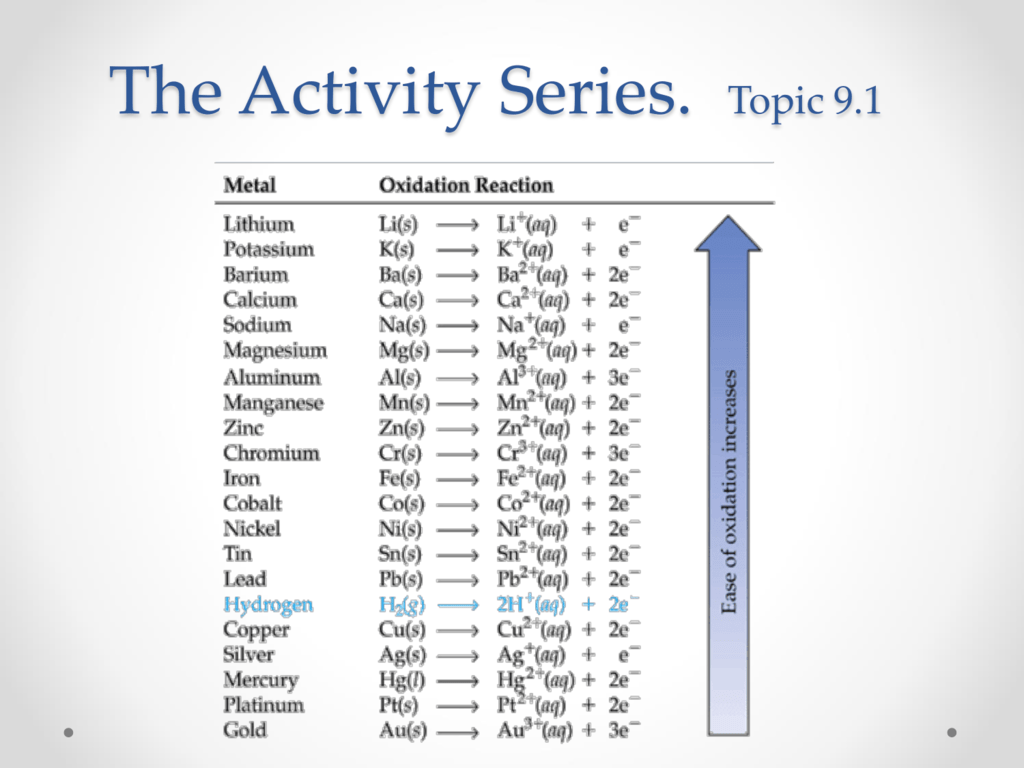
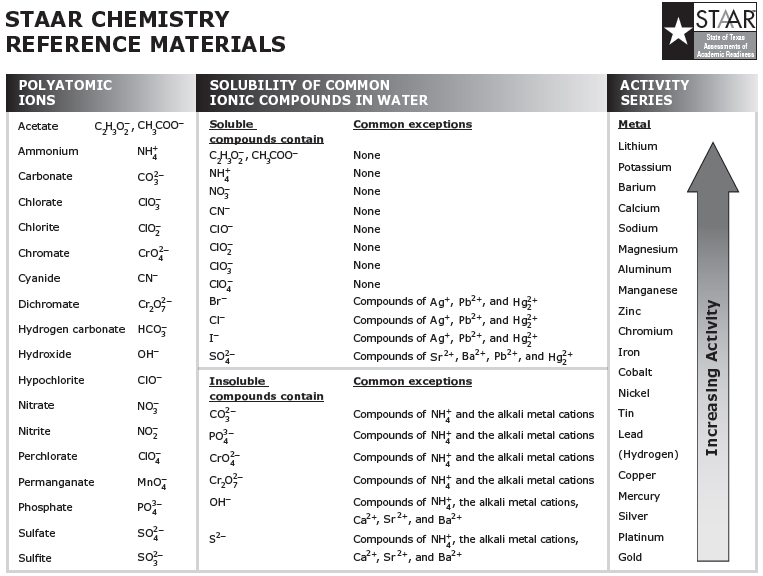
Topic 9.1 Activity Series

Activity Series Chemistry Video Clutch Prep
Exam Tips How to Score A in CIE AS and A Level Chemistry Malaysia
Chemistry Mr. Katmer's Chemistry Class

The Activity Series Pathways to Chemistry
Web Activity Series Of Metals;
Since Metals Replace Other Metals, While Nonmetals Replace Other Nonmetals, They Each Have A Separate Activity Series.
Write An Activity Series For 3 Or More Elements Based On A Multiple Replacement Reactions.
The Metals At The Top Of The Table, Especially The Alkali And Alkali Earth Metals, Are The Most Easily Oxidized.
Related Post: