Activity Series Chart
Activity Series Chart - Web 9 share 613 views 3 years ago chemistry science is for everyone! Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Web the activity series is a list of elements in decreasing order of their reactivity. The metals at the top of the table, especially the alkali and alkali earth metals, are the most easily oxidized. The top metals are more reactive than the metals on the bottom. In today's video, we are looking at how to use an activity series chart for metals and nonmetals. The table below is an activity series of. Define activity and determine which element in a reaction is more active given the balanced chemical equation. Specifically, use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction. It can also be used to obtain information on the reactivity of metals towards water and acids. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Web an activity series is a list of metals arranged in order of decreasing ease of oxidation. A chart of the reactivity series of common metals is provided below. They are called active metals. The top metals are more. Web writing equations to make an activity series. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) An activity series chart is an. A chart of the reactivity series of common metals is provided below. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by. Web the activity series of metals or reactivity series is a list of metals from most reactive to least reactive. The top metals are more reactive than the metals on the bottom. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) For example, both magnesium and zinc can react with hydrogen ions to displace. Knowing the activity series helps you predict whether or not a chemical reaction occurs. They are called active metals. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) A chart of the reactivity series of common metals is provided below. Web writing equations to make an activity series. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Web 9 share 613 views 3 years ago chemistry science is for everyone! Web the activity series is a list of elements in decreasing order of their reactivity. A chart of the reactivity series of common metals is provided below. The metals at the top. Define activity and determine which element in a reaction is more active given the balanced chemical equation. Web the activity series of metals or reactivity series is a list of metals from most reactive to least reactive. A chart of the reactivity series of common metals is provided below. The table \(\pageindex{1}\) below is an activity series of most common. The table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Web the activity series is a. Write an activity series for 3 or more elements based on a multiple replacement reactions. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Web an activity series is a list of metals arranged in order of decreasing ease of oxidation. Web the activity series is a list. Web the activity series is a chart of metals listed in order of declining relative reactivity. The metals at the top of the table, especially the alkali and alkali earth metals, are the most easily oxidized. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: A chart of. An activity series chart is an. Web the activity series is a list of elements in decreasing order of their reactivity. Knowing the activity series helps you predict whether or not a chemical reaction occurs. The table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the. Web the data. Web in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and structurally analytical progression [1] of a series of metals, arranged by their reactivity from highest to lowest. The table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the. A chart of the reactivity series of common metals is provided below. Web the data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. Knowing the activity series helps you predict whether or not a chemical reaction occurs. It can also be used to obtain information on the reactivity of metals towards water and acids. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Web 9 share 613 views 3 years ago chemistry science is for everyone! Define activity and determine which element in a reaction is more active given the balanced chemical equation. Web writing equations to make an activity series. In today's video, we are looking at how to use an activity series chart for metals and nonmetals. The metals at the top of the table, especially the alkali and alkali earth metals, are the most easily oxidized. The table below is an activity series of. They are called active metals. Web the activity series is a list of elements in decreasing order of their reactivity.
Activity Series Chemistry Video Clutch Prep
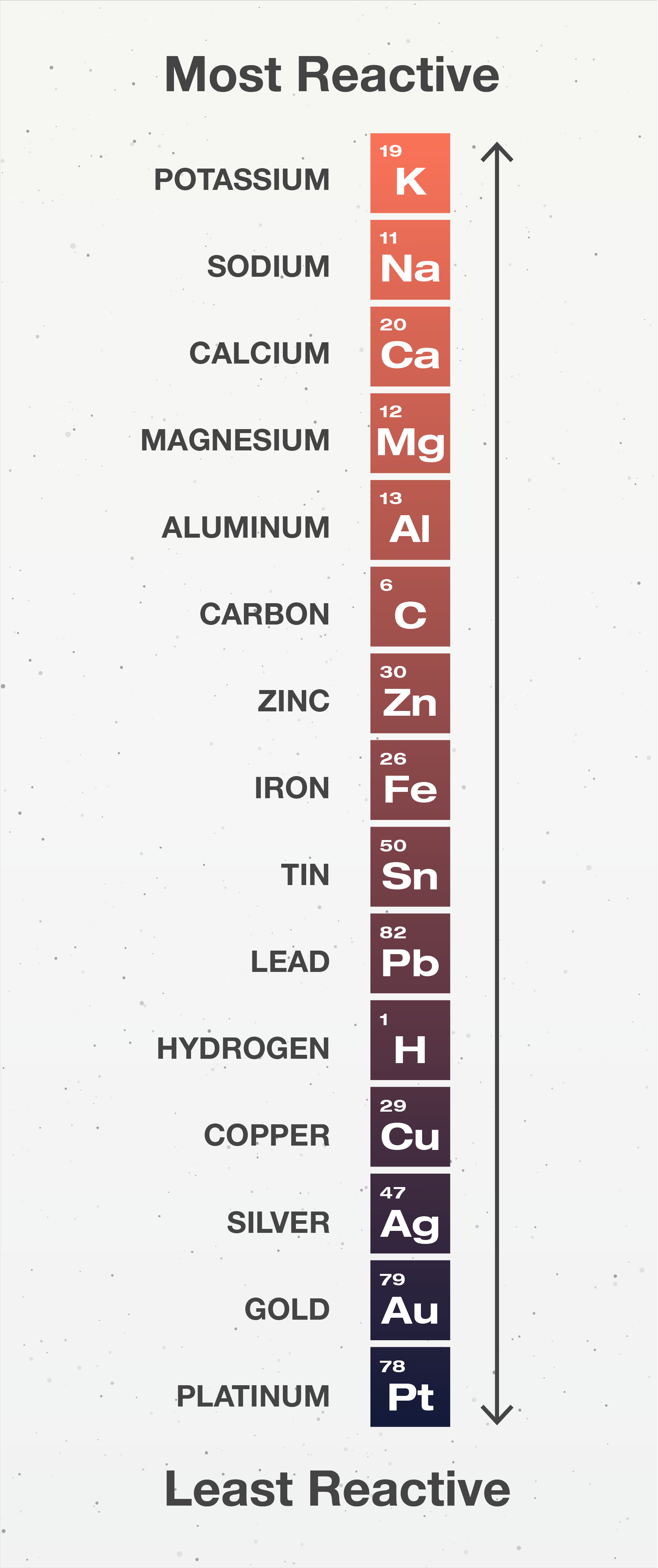
5.1 Activity Series Chemistry LibreTexts

Oxidation Number of Chromium AnnieqoIngram

The Activity Series Pathways to Chemistry
Activity Series Chart PDF
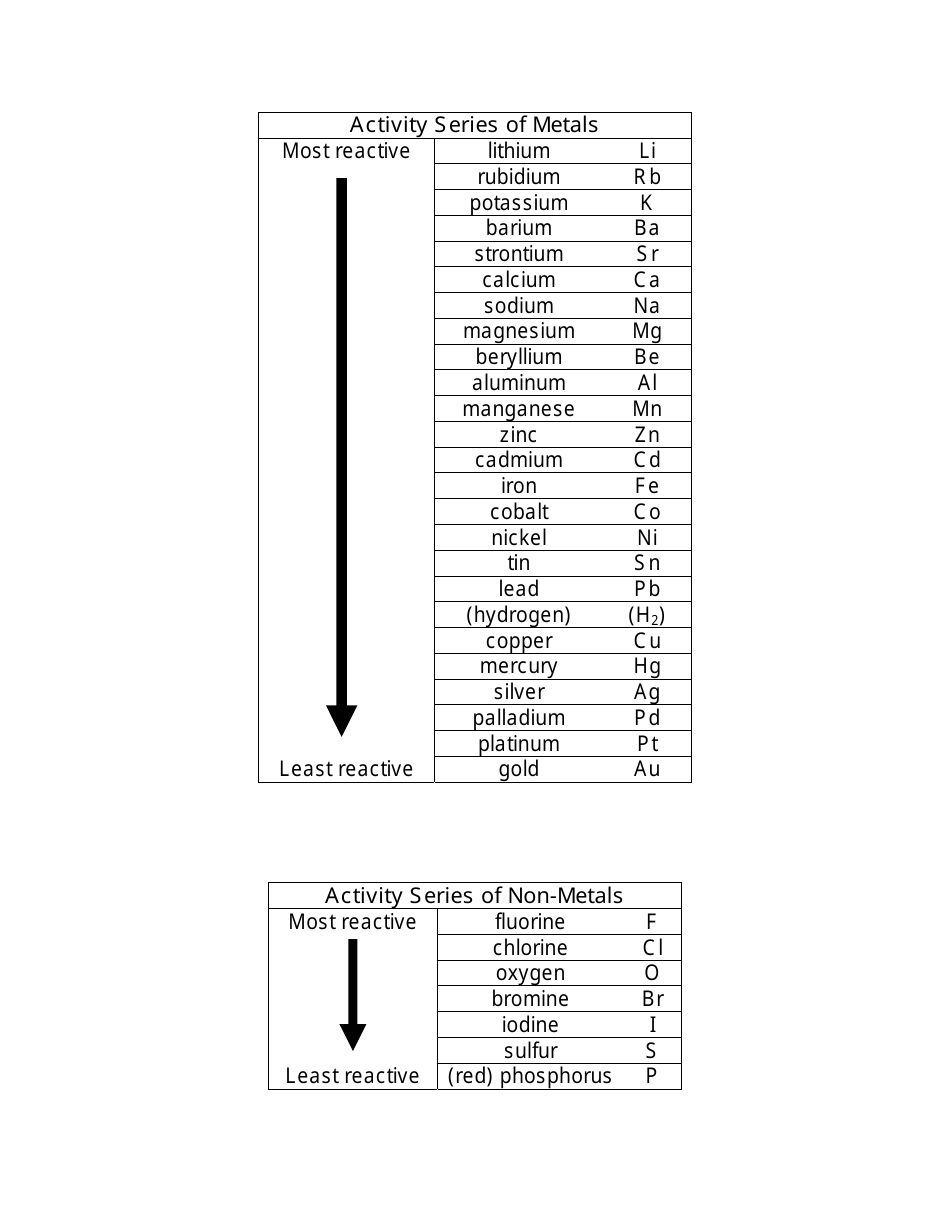
Activity Series of Metals and Nonmetals Cheat Sheet Download Printable

Activity Series of Metals (Reactivity Series)

The Activity Series YouTube

The Activity Series YouTube
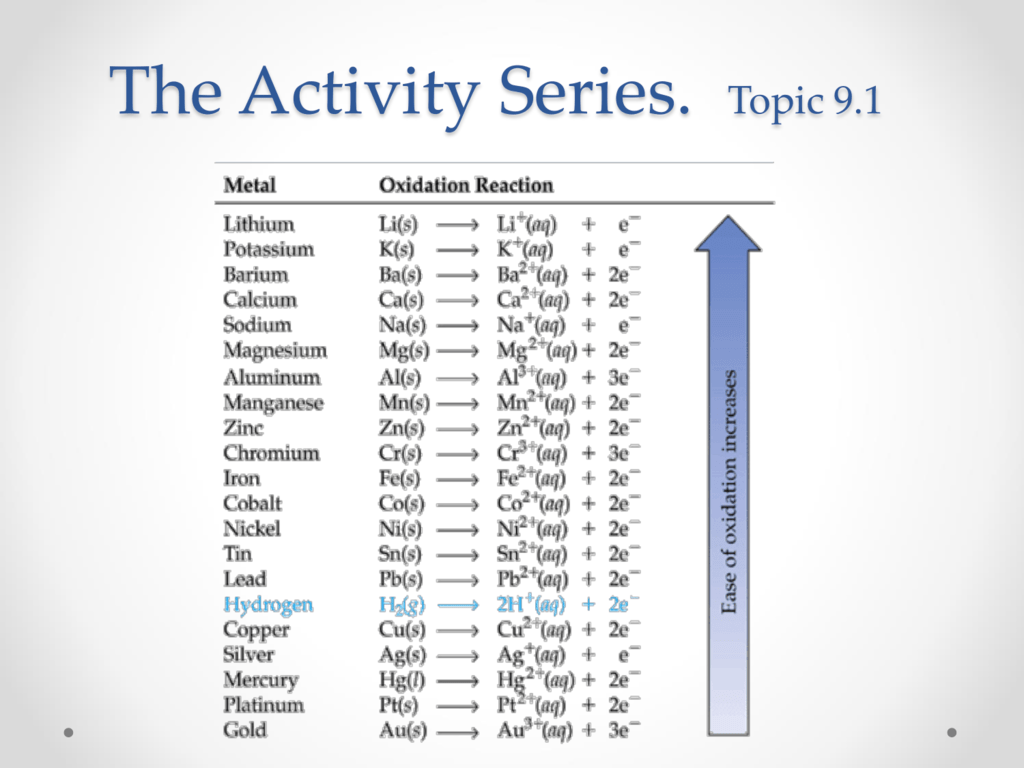
Topic 9.1 Activity Series
Web An Activity Series Is A List Of Metals Arranged In Order Of Decreasing Ease Of Oxidation.
Since Metals Replace Other Metals, While Nonmetals Replace Other Nonmetals, They Each Have A Separate Activity Series.
Web The Activity Series Is A Chart Of Metals Listed In Order Of Declining Relative Reactivity.
Since Metals Replace Other Metals, While Nonmetals Replace Other Nonmetals, They Each Have A Separate Activity Series.
Related Post:
