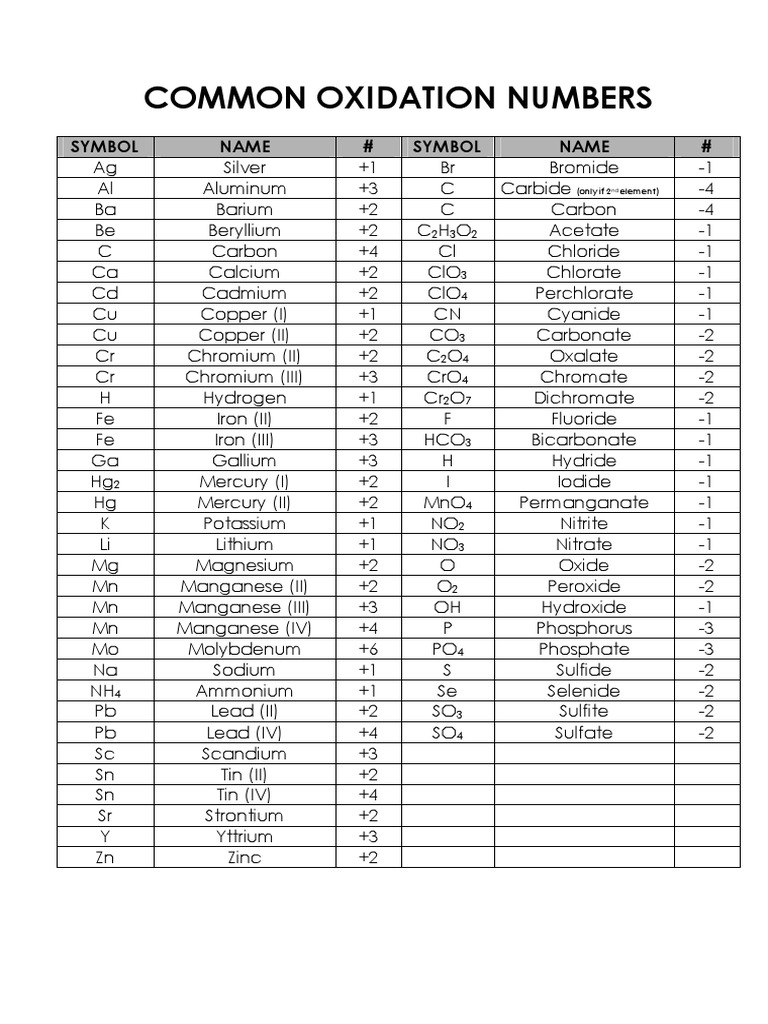
Chart Of Oxidation Number
Chart Of Oxidation Number - The oxidation number, in simple terms, can be described as the number that is allocated to elements in a chemical combination. Web using a list of simple rules you’ll learn how to find the oxidation numbers for elements and compounds. Explore book buy on amazon. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web make use of this chart to know the common , positive and negative oxidation states of common elements. The oxidation number of zn, al, h 2, o 2, and cl 2 is zero. This oxidation number concept was. You can also see the common and uncommon oxidation states of each element, and the net ionic. Web enter a chemical formula to calculate the oxidation numbers of the elements. For each rule there are examples and practice calcul. The oxidation number of an element in its free state is zero. Web enter a chemical formula to calculate the oxidation numbers of the elements. Oxidation numbers are bookkeeping numbers. Web the sum of the oxidation numbers in a polyatomic ion is equal to the charge on that ion. Oxidation number when an element has not combined or do not. When an element has not combined, it's oxidation number is 0. For each rule there are examples and practice calcul. Web using a list of simple rules you’ll learn how to find the oxidation numbers for elements and compounds. The oxidation number of a monatomic. Web the sum of the oxidation numbers in a polyatomic ion is equal to the. Web the sum of the oxidation numbers in a polyatomic ion is equal to the charge on that ion. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. Web 60 rows find the oxidation numbers of monoatomic ions for each element in the. Web the oxidation number is the total number of. Oxidation numbers are bookkeeping numbers. For example, the oxidation numbers of \(\ce{k^+}\),. In order to learn how to find the oxidation number of an atom in a. Web the oxidation number represents how many electrons an atom has gained or lost in a molecule. This oxidation number concept was. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web oxidation number is a positive or negative number assigned to an atom according to a set of rules. Web the oxidation number is the total number of electrons that atoms in a molecule can share, lose, or acquire. Web the oxidation number represents how many electrons an atom has gained or lost in a molecule. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. 0 , +2 , +4 , +7. The oxidation number of an element in its free state is zero. Download a pdf chart of common oxidation. Web make use of this chart to know the common , positive and negative oxidation states of common elements. Oxidation numbers are bookkeeping numbers. Web oxidation number is a positive or negative number assigned to an atom according to a set of rules. The atoms in he and n 2, for example, have oxidation numbers of 0. For example, the. The atoms in he and n 2, for example, have oxidation numbers of 0. Web using a list of simple rules you’ll learn how to find the oxidation numbers for elements and compounds. Web list of oxidation states of the elements. 0 , +2 , +4 , +7. The oxidation number is also called as the oxidation state. The oxidation number of zn, al, h 2, o 2, and cl 2 is zero. The oxidation number is also called as the oxidation state. Explore book buy on amazon. Web the sum of the oxidation numbers in a polyatomic ion is equal to the charge on that ion. For each rule there are examples and practice calcul. Web list of oxidation states of the elements. In order to learn how to find the oxidation number of an atom in a. Web in the nomenclature of inorganic chemistry, the oxidation number of an element that may exist in more than one oxidation state is indicated by a roman numeral. Elements have an oxidation state of zero, and atoms. When an element has not combined, it's oxidation number is 0. Web list of oxidation states of the elements. Web the oxidation number represents how many electrons an atom has gained or lost in a molecule. Web make use of this chart to know the common , positive and negative oxidation states of common elements. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. How to find oxidation number? The oxidation number is also called as the oxidation state. This oxidation number concept was. The oxidation number of zn, al, h 2, o 2, and cl 2 is zero. Again, work backwards to determine the oxidation number of any. Web 60 rows find the oxidation numbers of monoatomic ions for each element in the. Oxidation number when an element has not combined or do not form a compound. Oxidation numbers are bookkeeping numbers. How to calculate oxidation number. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web in the nomenclature of inorganic chemistry, the oxidation number of an element that may exist in more than one oxidation state is indicated by a roman numeral.
Downloadable Periodic Table Oxidation States
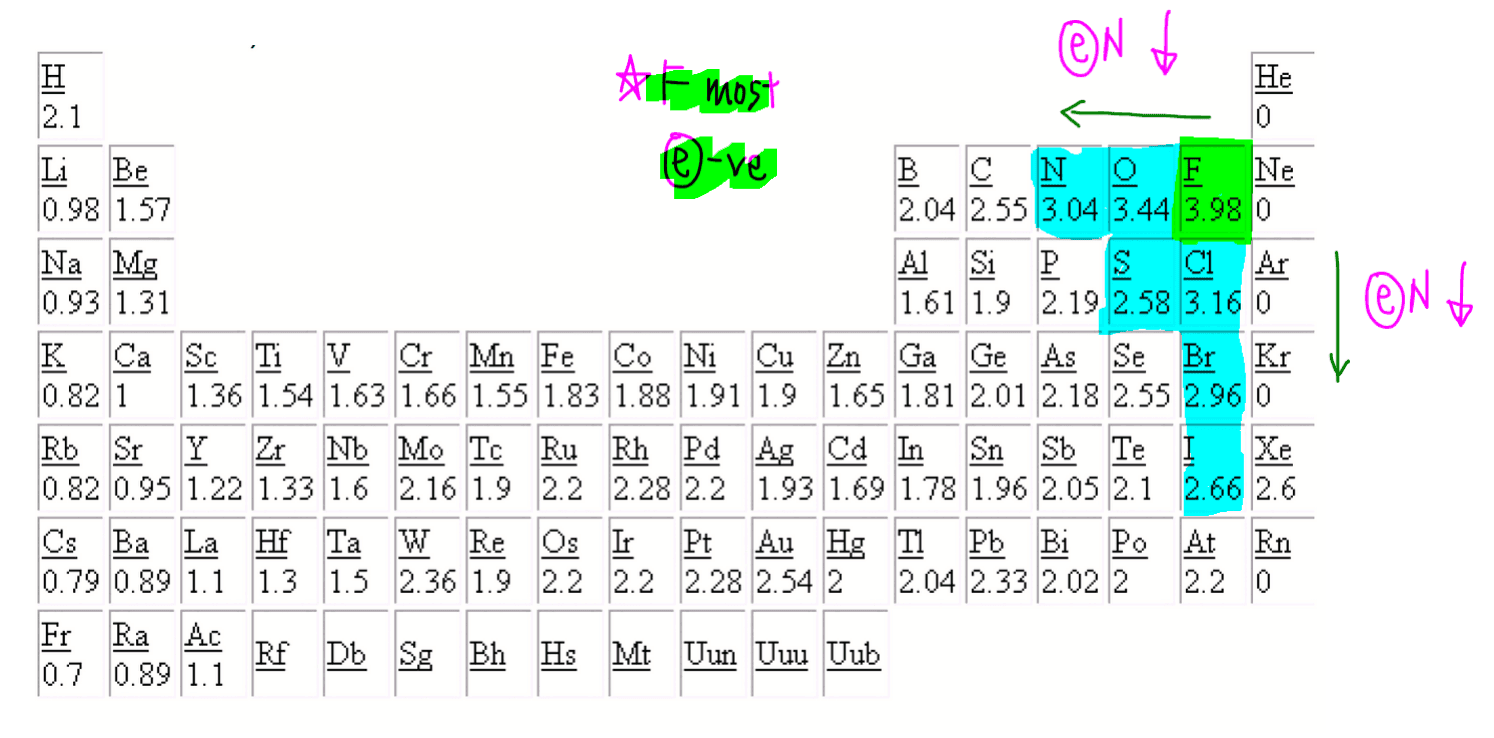
What is Oxidation State?

Pin by Natalie Paes on chem Chemistry education, Chemical science, Teaching chemistry
Common Oxidation Numbers Chart
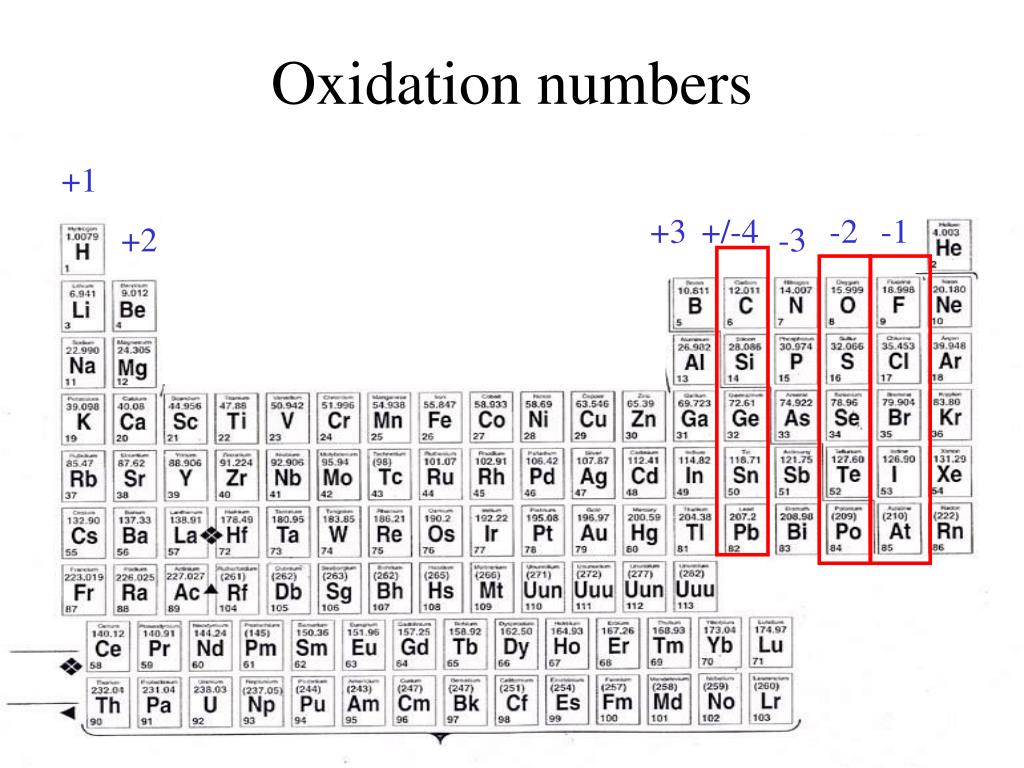
Periodic Table Oxidation Chart
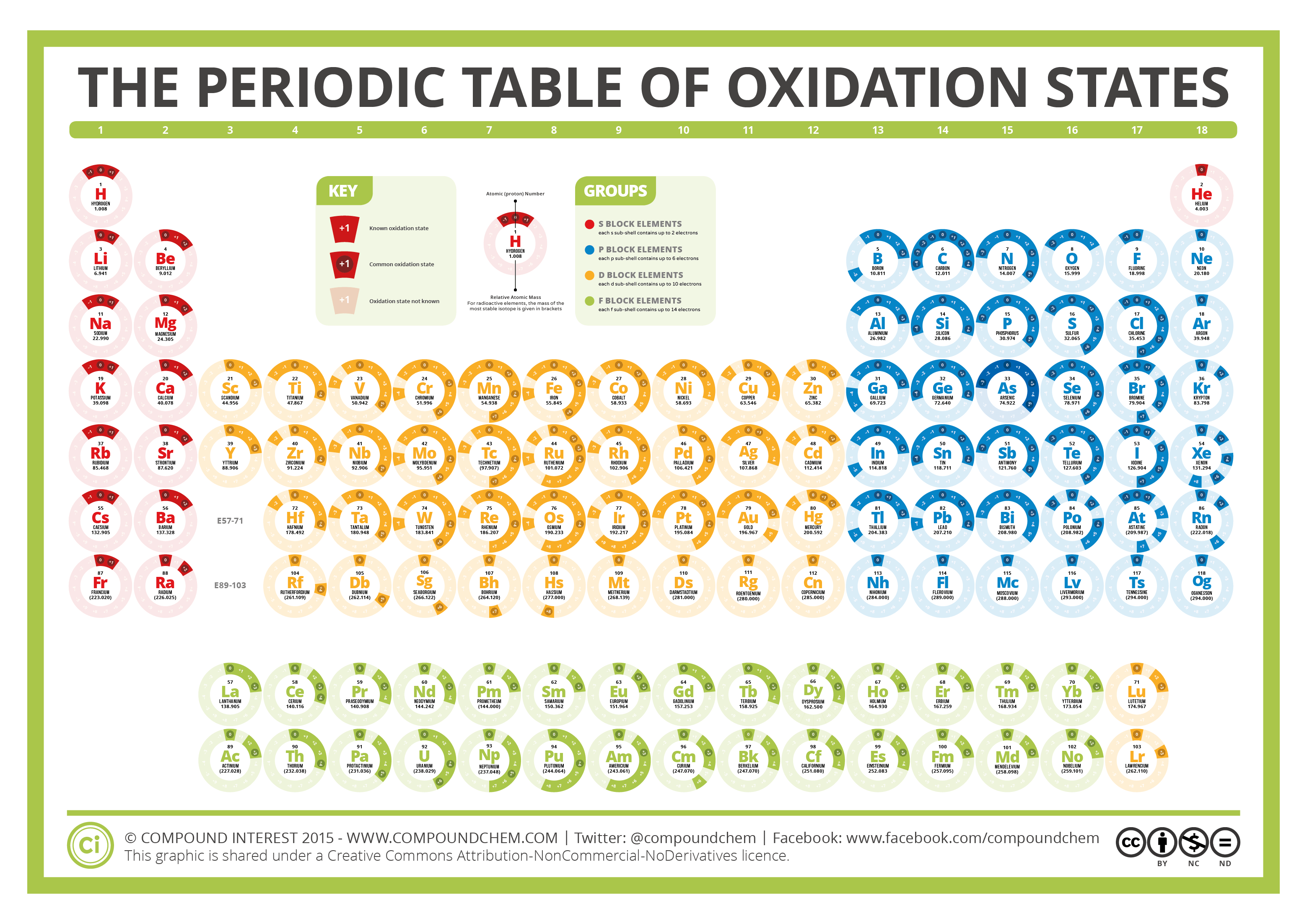
The Periodic Table of Oxidation States Compound Interest
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers

Periodic Table With Oxidation Numbers And Names Elcho Table The Best Porn Website
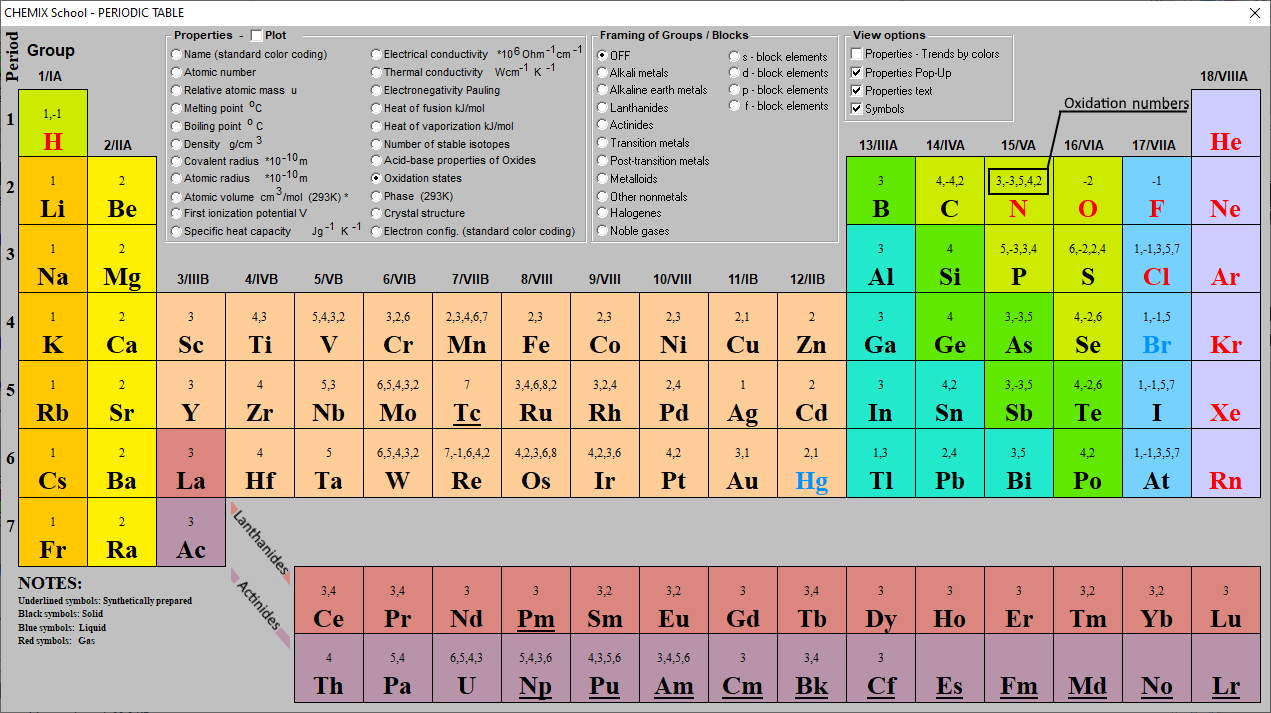
Oxidation Number Periodic Table Elements Definition, Rules, 54 OFF
the periodic table of oxidation states compound interest 65 info periodic table elements
For Each Rule There Are Examples And Practice Calcul.
Download A Pdf Chart Of Common Oxidation.
In Order To Learn How To Find The Oxidation Number Of An Atom In A.
Web The Sum Of The Oxidation Numbers In A Polyatomic Ion Is Equal To The Charge On That Ion.
Related Post: