Enthalpy Chart
Enthalpy Chart - Web calculate the enthalpy of formation for acetylene, c 2 h 2 (g) from the combustion data (table 5.7.1, note acetylene is not on the table) and then compare your answer to the value in table 5.7.2. Evaluating an enthalpy of formation. Web calculate enthalpy changes for various chemical reactions. Web the enthalpy values of solid aluminum, beryllium, gold, and copper are zero, but the vapor phases of these metals do have enthalpy values. When you reverse the direction of a chemical reaction, the magnitude of δh is the same, but the sign changes. 1 illustrates some of these definitions, using water as the medium experiencing a heat transfer process. The enthalpy of the water (heat content in btu/lb). [1] it is a state function used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. Explain hess’s law and use it to compute reaction enthalpies. Web in thermodynamics, enthalpy / ˈɛnθəlpi / ⓘ, is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. We all know that water boils at 212of (atmospheric pressure at sea level). This graph plots the water temperature vs. 1 illustrates some of these definitions, using water as the medium experiencing a heat transfer process. Explain hess’s law and use it to compute reaction enthalpies. Web calculate the enthalpy of formation for acetylene, c 2 h 2 (g) from. Explain hess’s law and use it to compute reaction enthalpies. When you reverse the direction of a chemical reaction, the magnitude of δh is the same, but the sign changes. Evaluating an enthalpy of formation. 1 illustrates some of these definitions, using water as the medium experiencing a heat transfer process. This graph plots the water temperature vs. We all know that water boils at 212of (atmospheric pressure at sea level). Thermochemistry is a branch of chemical thermodynamics, the science that deals with the relationships between heat, work, and other forms of energy in the context of chemical and physical processes. When you reverse the direction of a chemical reaction, the magnitude of δh is the same, but. 1 illustrates some of these definitions, using water as the medium experiencing a heat transfer process. This graph plots the water temperature vs. Web in thermodynamics, enthalpy / ˈɛnθəlpi / ⓘ, is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. Ozone, o 3 ( g ), forms from oxygen, o 2. We all know that water boils at 212of (atmospheric pressure at sea level). Web by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for gases and 1 m for solutions. Ozone, o 3 ( g ), forms from oxygen, o 2 ( g. Web the enthalpy values of solid aluminum, beryllium, gold, and copper are zero, but the vapor phases of these metals do have enthalpy values. Web calculate the enthalpy of formation for acetylene, c 2 h 2 (g) from the combustion data (table 5.7.1, note acetylene is not on the table) and then compare your answer to the value in table. We all know that water boils at 212of (atmospheric pressure at sea level). The enthalpy of the water (heat content in btu/lb). Web in thermodynamics, enthalpy / ˈɛnθəlpi / ⓘ, is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. This graph plots the water temperature vs. Web calculate the enthalpy of. Web by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for gases and 1 m for solutions. [1] it is a state function used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by. 1 illustrates some of these definitions, using water as the medium experiencing a heat transfer process. Web calculate enthalpy changes for various chemical reactions. Explain hess’s law and use it to compute reaction enthalpies. Web in thermodynamics, enthalpy / ˈɛnθəlpi / ⓘ, is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume.. Web calculate enthalpy changes for various chemical reactions. Web calculate the enthalpy of formation for acetylene, c 2 h 2 (g) from the combustion data (table 5.7.1, note acetylene is not on the table) and then compare your answer to the value in table 5.7.2. We all know that water boils at 212of (atmospheric pressure at sea level). Explain hess’s. Explain hess’s law and use it to compute reaction enthalpies. Web in thermodynamics, enthalpy / ˈɛnθəlpi / ⓘ, is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. Web calculate enthalpy changes for various chemical reactions. The enthalpy of the water (heat content in btu/lb). Ozone, o 3 ( g ), forms from oxygen, o 2 ( g ), by an endothermic process. Web by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for gases and 1 m for solutions. Evaluating an enthalpy of formation. This graph plots the water temperature vs. Web the enthalpy values of solid aluminum, beryllium, gold, and copper are zero, but the vapor phases of these metals do have enthalpy values. [1] it is a state function used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. Web calculate the enthalpy of formation for acetylene, c 2 h 2 (g) from the combustion data (table 5.7.1, note acetylene is not on the table) and then compare your answer to the value in table 5.7.2. We all know that water boils at 212of (atmospheric pressure at sea level).
Pressure Enthalpy Chart
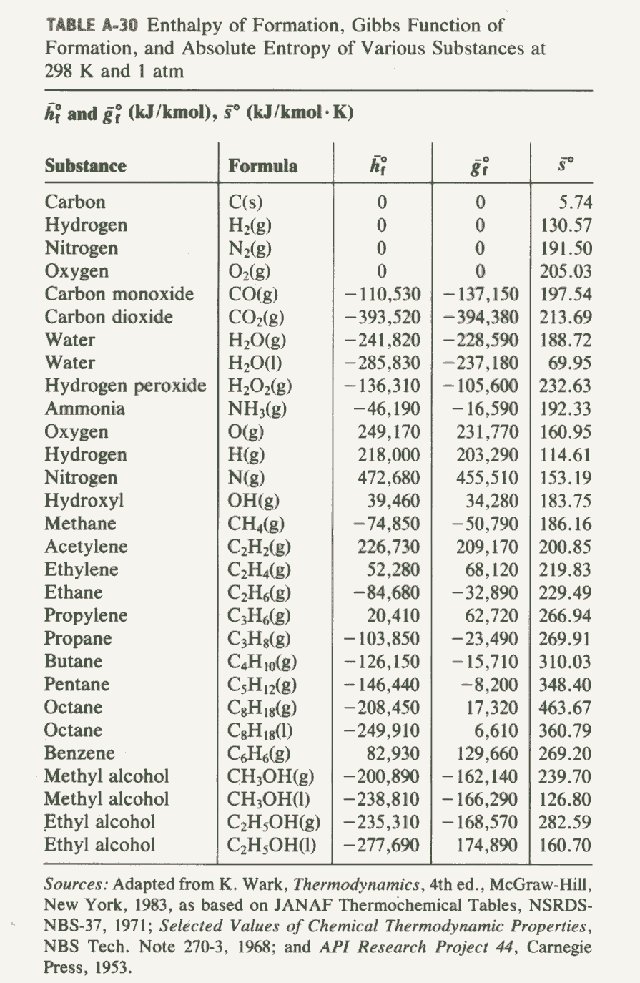
Sampoerna Wallpaper enthalpy of formation
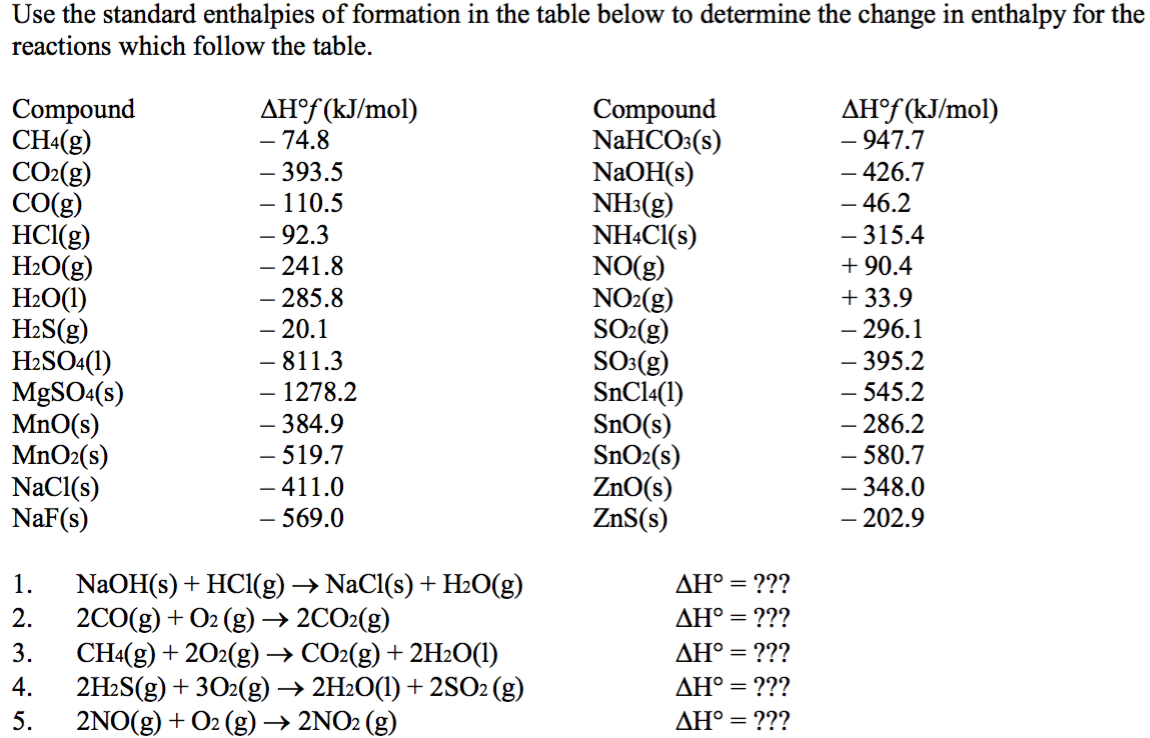
Solved Use the standard enthalpies of formation in the table

Enthalpy Charts What They Can Tell the Technician InstruMetriX® Test

Pressure Enthalpy Chart

CO2 as a Refrigerant — Introduction to Transcritical Operation

Pressure / Enthalpy Diagram Example HVAC School
[Solved] choose all that apply which have enthalpies of combustion that

Enthalpy Diagram Diagram Site
EnthalpyEntropy Diagram (Air) Enthalpy Statistical Mechanics
When You Reverse The Direction Of A Chemical Reaction, The Magnitude Of Δh Is The Same, But The Sign Changes.
Thermochemistry Is A Branch Of Chemical Thermodynamics, The Science That Deals With The Relationships Between Heat, Work, And Other Forms Of Energy In The Context Of Chemical And Physical Processes.
1 Illustrates Some Of These Definitions, Using Water As The Medium Experiencing A Heat Transfer Process.
Related Post:
