Galvanic Action Chart
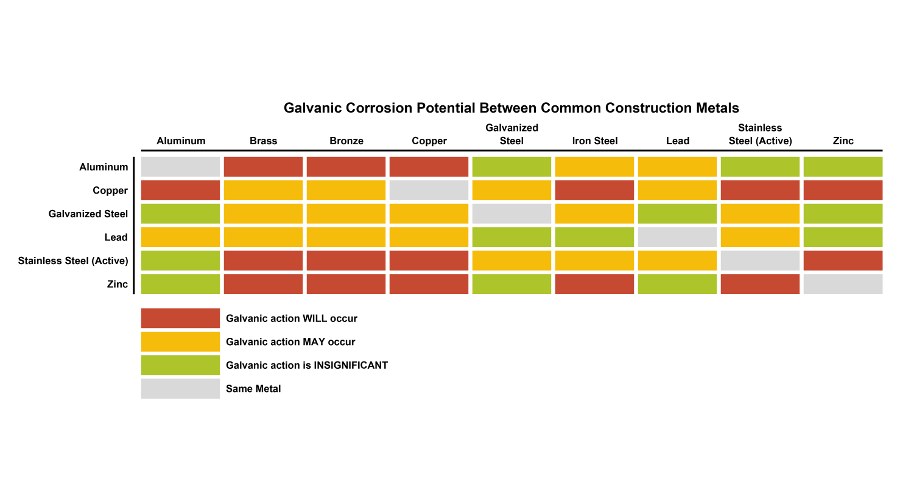
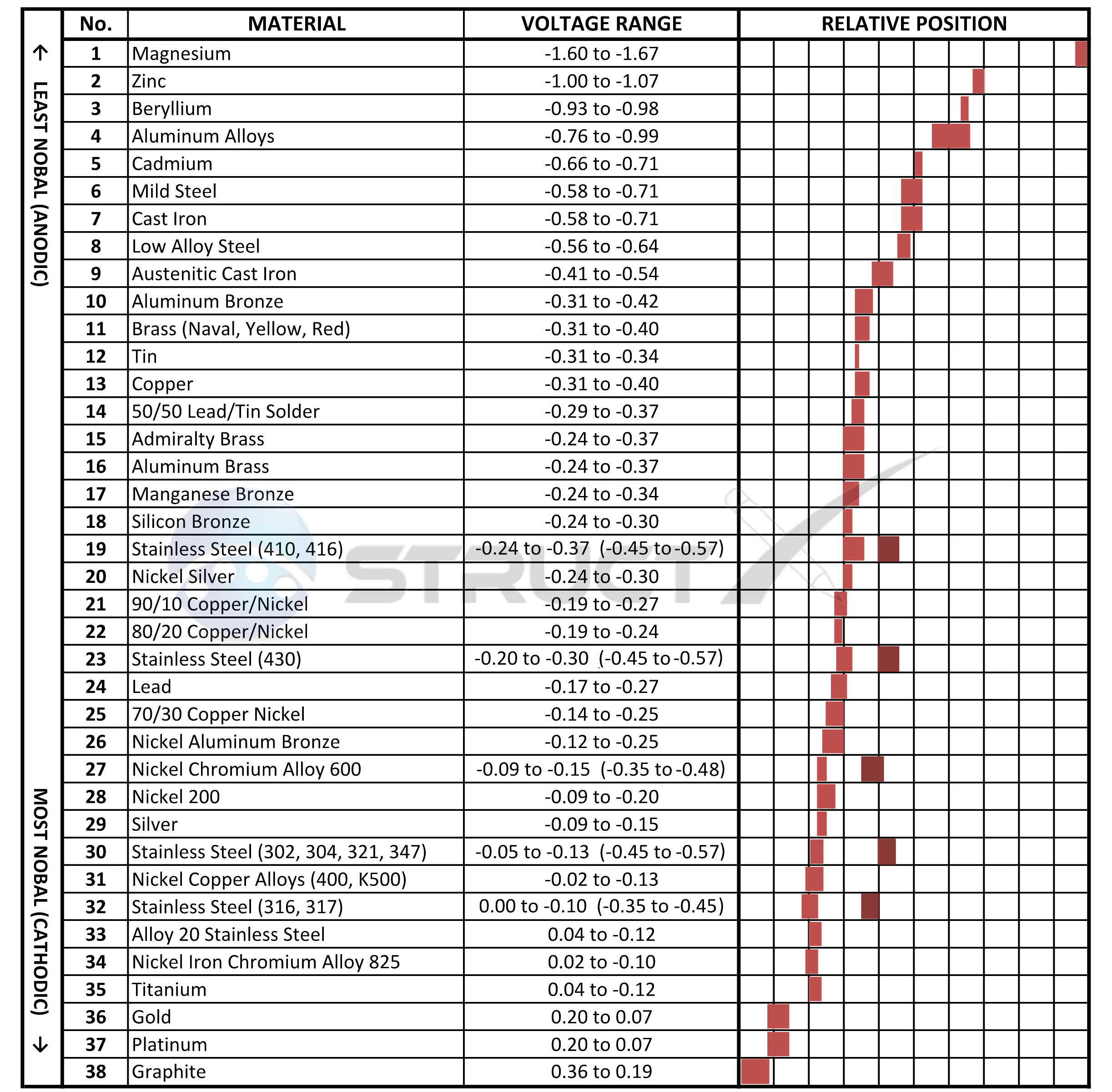
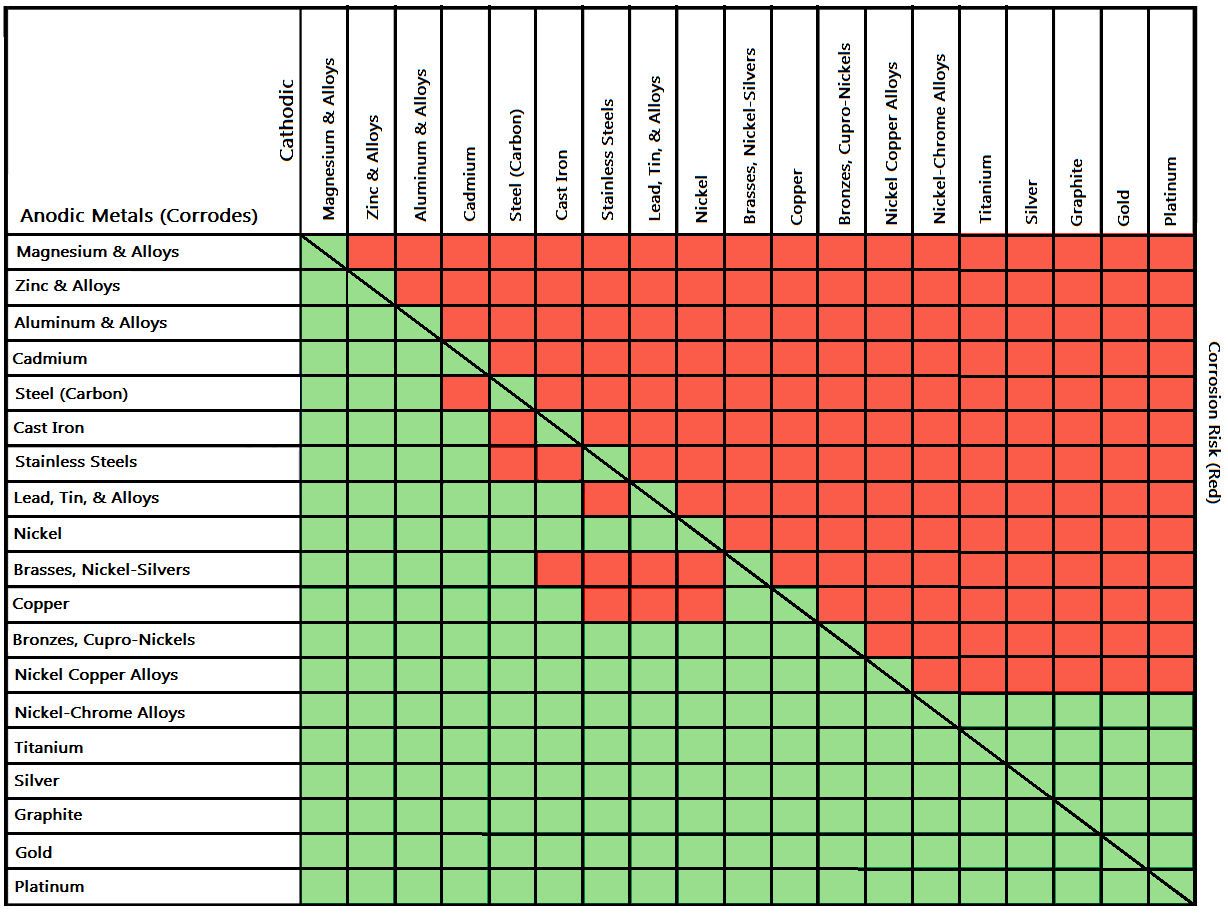
Galvanic Action Chart - The unshaded symbols show ranges exhibited by stainless steels in acidic water such as may exist in crevices or in. Stainless steel series 400 ↔ 7. There are three conditions that must exist for galvanic corrosion to occur. Iron anodic or active (+) 5. Web result a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. Web result most designers know enough to be dangerous, but how exactly does galvanic corrosion work, and what are the best practices for preventing it? We also provide other helpful methods for. Web result chart 1. First there must be two electrochemically dissimilar metals present. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the contact metal listed in the upper row; Values given here are for indicative purpose only. First there must be two electrochemically dissimilar metals present. For galvanic corrosion to occur, four elements are necessary: Web result the following graph is for a galvanic series in simulated seawater. Web result figure 1: Web result by simply choosing metals that avoid galvanic action there will never be an issue. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a. Web result up a galvanic action which results in the deterioration of one of them. This series of alloys assumes full submersion in flowing seawater. Web result below,. Web result the following graph is for a galvanic series in simulated seawater. Web result this slide includes a chart of galvanic corrosion potential between common construction metals. The following is a list of the more common commercial metals, sequenced according to what is known as the galvanic table: Web result galvanic corrosion chart. Unless noted, the data was acquired. Stainless steel series 400 ↔ 7. Web result up a galvanic action which results in the deterioration of one of them. In application, these metals can exhibit a range of voltage potential relative to a reference electrode. A “galvanic series” applies to a particular electrolyte solution, hence for each. Though the order of metals in a galvanic series remains the. (noble metals are those that are resistant to corrosion and oxidation.) when two metals are immersed in an electrolyte, while also being connected externally by a conductor, the less noble metal experiences. Web result the following galvanic table lists metals in the order of their relative activity in seawater environment. Web result figure 1: Web result a chart depicting the. The list begins with the more active (anodic) metal and proceeds down the to the least active (cathodic) metal of the galvanic series. In this respect, one should understand how to read the following chart which lists all metals. Web result this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web result galvanic corrosion process. Web result the following galvanic table lists metals in the order of their relative activity in seawater environment. Web result galvanic corrosion chart. View our galvanic series chart, pictures, definitions,. Web result galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web result chart 1. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Web result. Web result this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. The list begins with the more active (anodic) metal and proceeds down the to the least active (cathodic) metal of the galvanic series. A “galvanic series” applies to a particular electrolyte solution, hence. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the contact metal listed in the upper row; (noble metals are those that are resistant to corrosion and oxidation.) when two metals are immersed in an electrolyte, while also being connected externally by a conductor, the less noble metal experiences.. Web result up a galvanic action which results in the deterioration of one of them. Web result this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web result below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. There are three conditions that must exist for galvanic corrosion to occur. Web result galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Below is a galvanic reaction chart for dissimilar metals. Web result by simply choosing metals that avoid galvanic action there will never be an issue. Web result galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web result however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web result galvanic corrosion chart. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web result this slide includes a chart of galvanic corrosion potential between common construction metals. In application, these metals can exhibit a range of voltage potential relative to a reference electrode. Unless noted, the data was acquired using the specifications set forth by astm 82 (standard guide for development and use of a galvanic series for electrochemical measurements in. To minimize galvanic corrosion, select fasteners based on their material compatibility with the. Web result chart 1.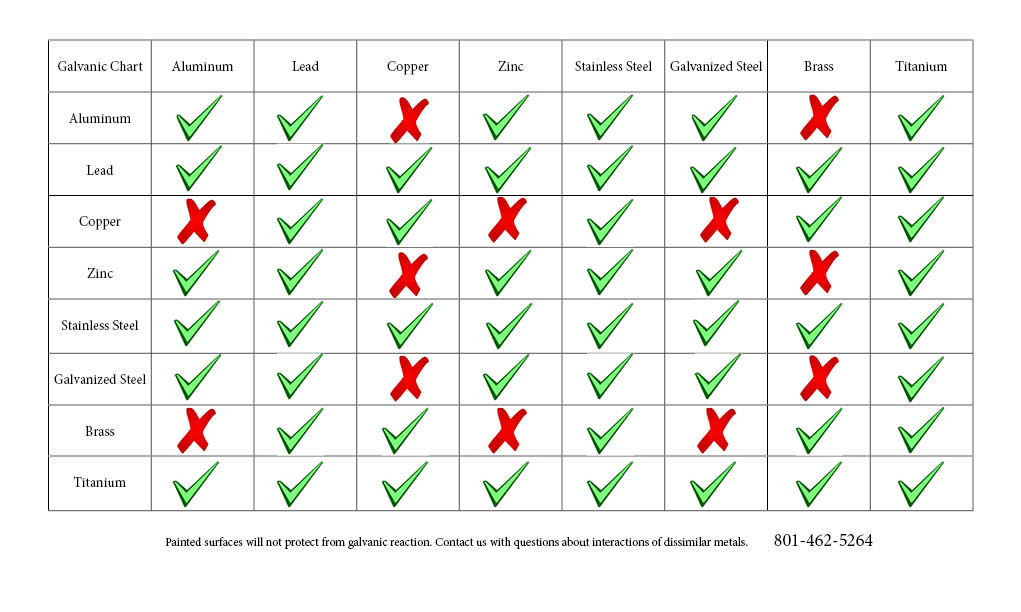
Attaching Stainless Steel to Aluminum DIY Home Improvement Forum
Beware of Galvanic Action (It's a Thing) 20180710 Building Enclosure
Instrumentation Tubing and Their Connections 1.0 Introduction to

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot

The Galvanic Series the essential guide EngineeringClicks
Trailer Cable Core Failure
Galvanic Corrosion Common Questions Answered

Beware of Galvanic Action (It's a Thing) 20180710 Building Enclosure

Galvanic Corrosion Chart Pay attention! You might accidentally learn
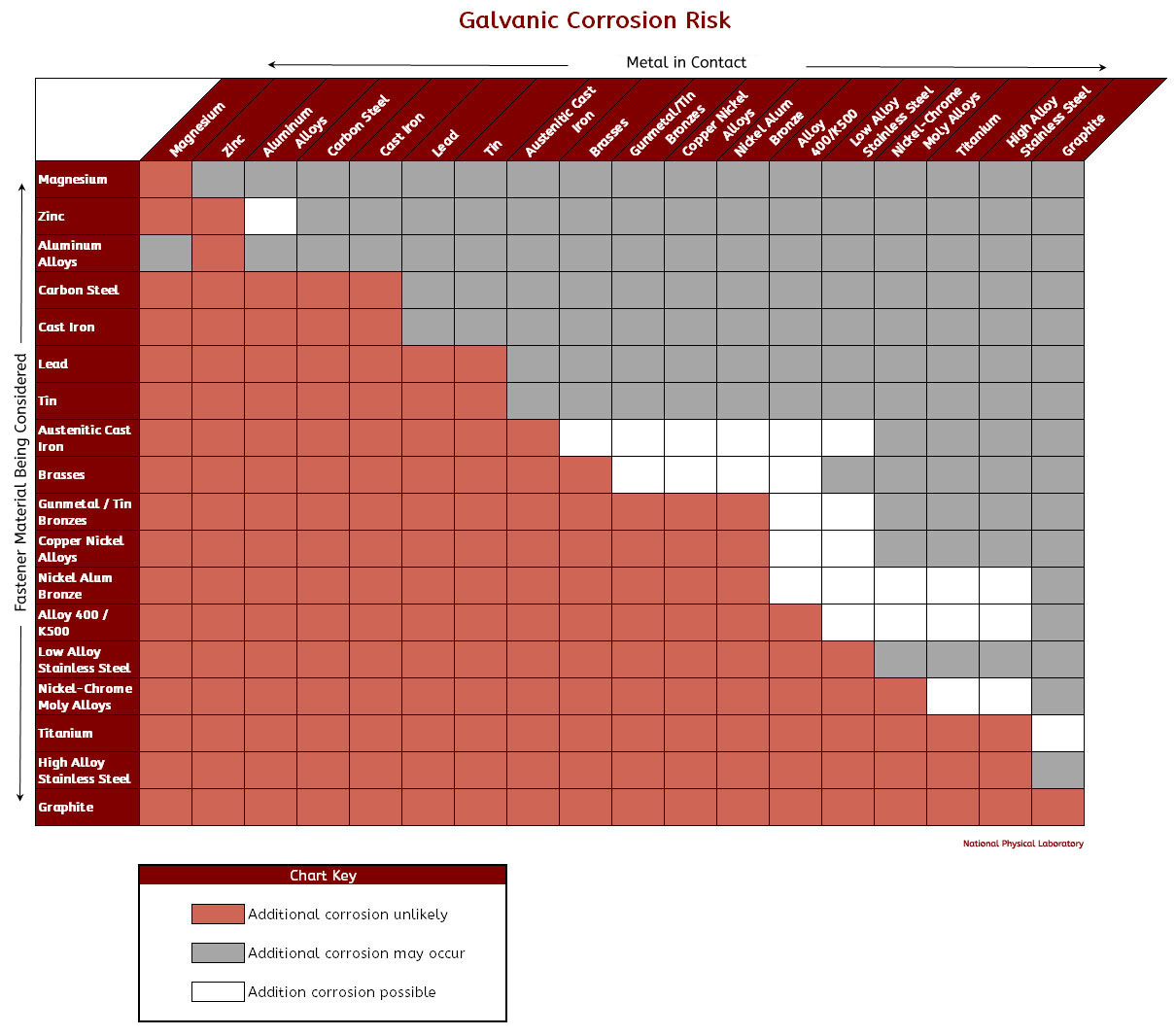
Galvanic Corrosion Scale
For Galvanic Corrosion To Occur, Four Elements Are Necessary:
To Use The Chart, Align The Metal To Be Assessed (For The Risk Of Corrosion) In The Left Column With The Contact Metal Listed In The Upper Row;
The Unshaded Symbols Show Ranges Exhibited By Stainless Steels In Acidic Water Such As May Exist In Crevices Or In.
Iron Anodic Or Active (+) 5.
Related Post: