Galvanic Reaction Chart
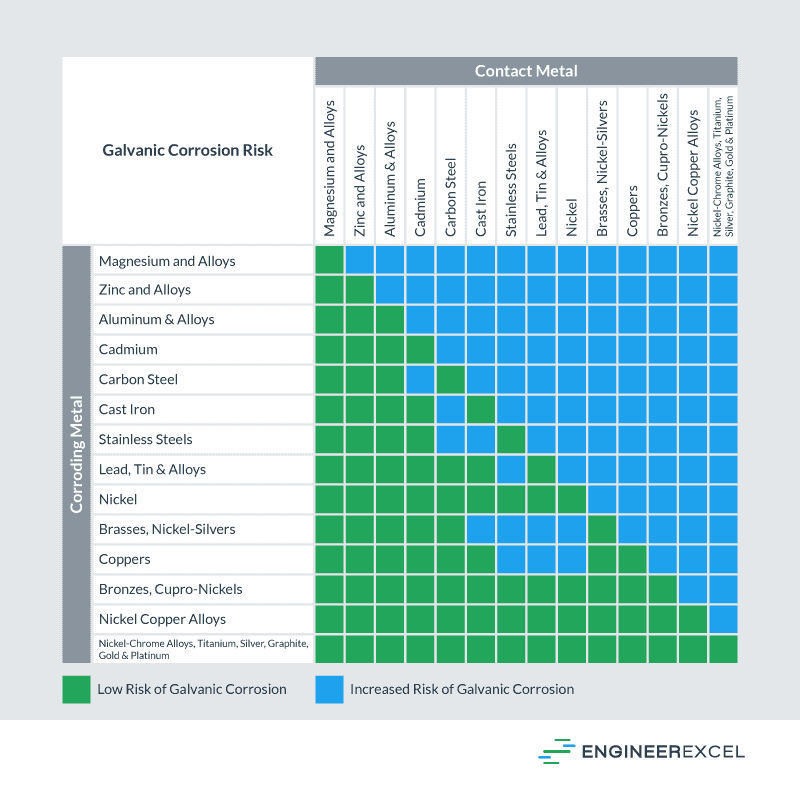
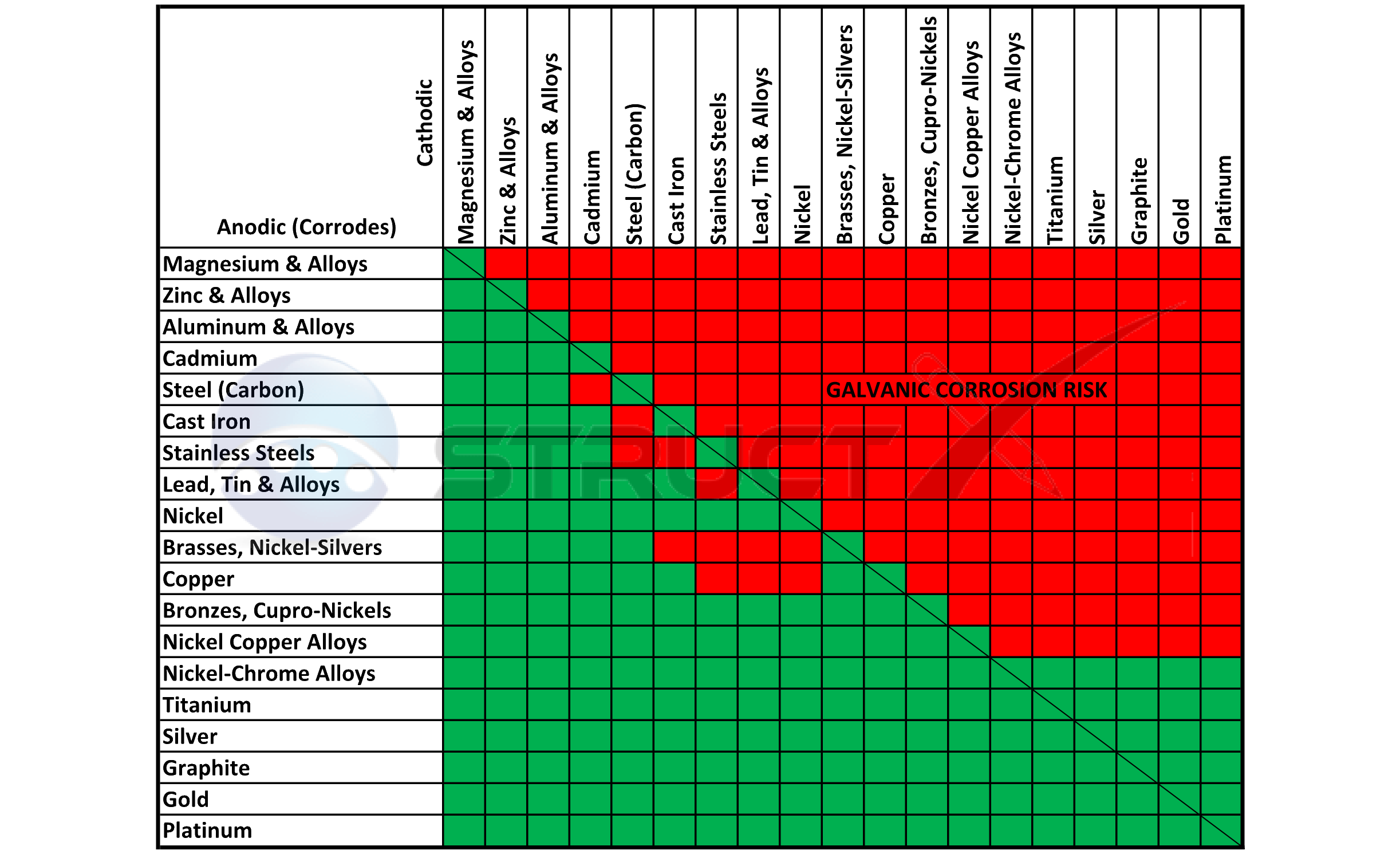
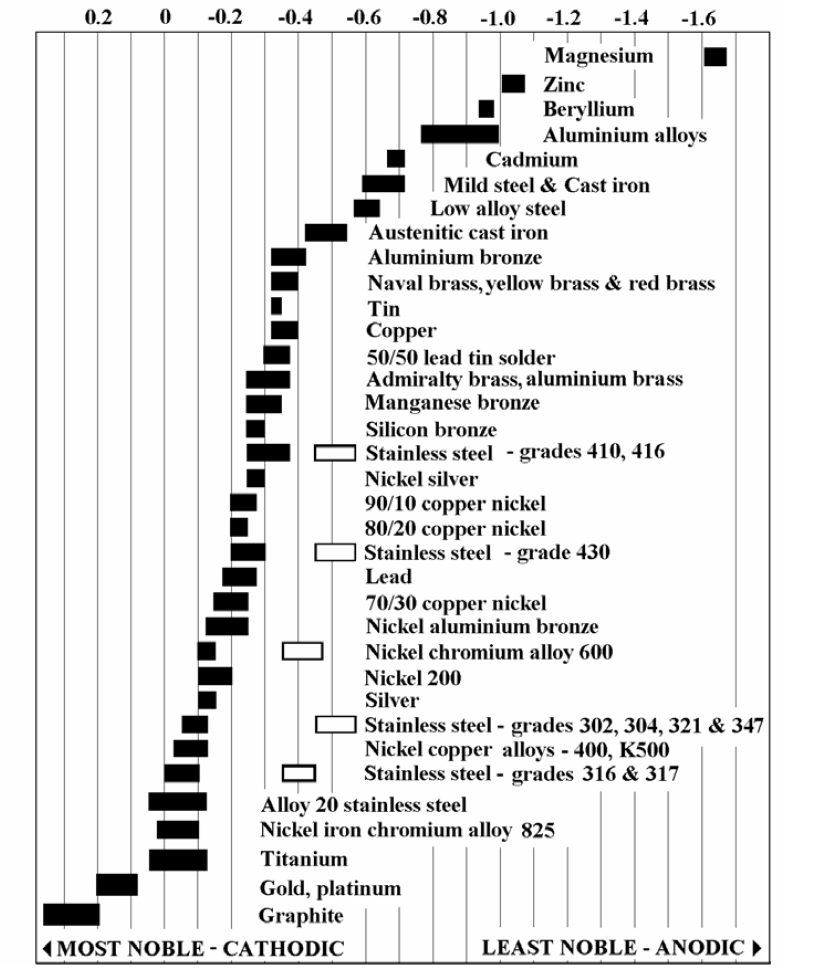
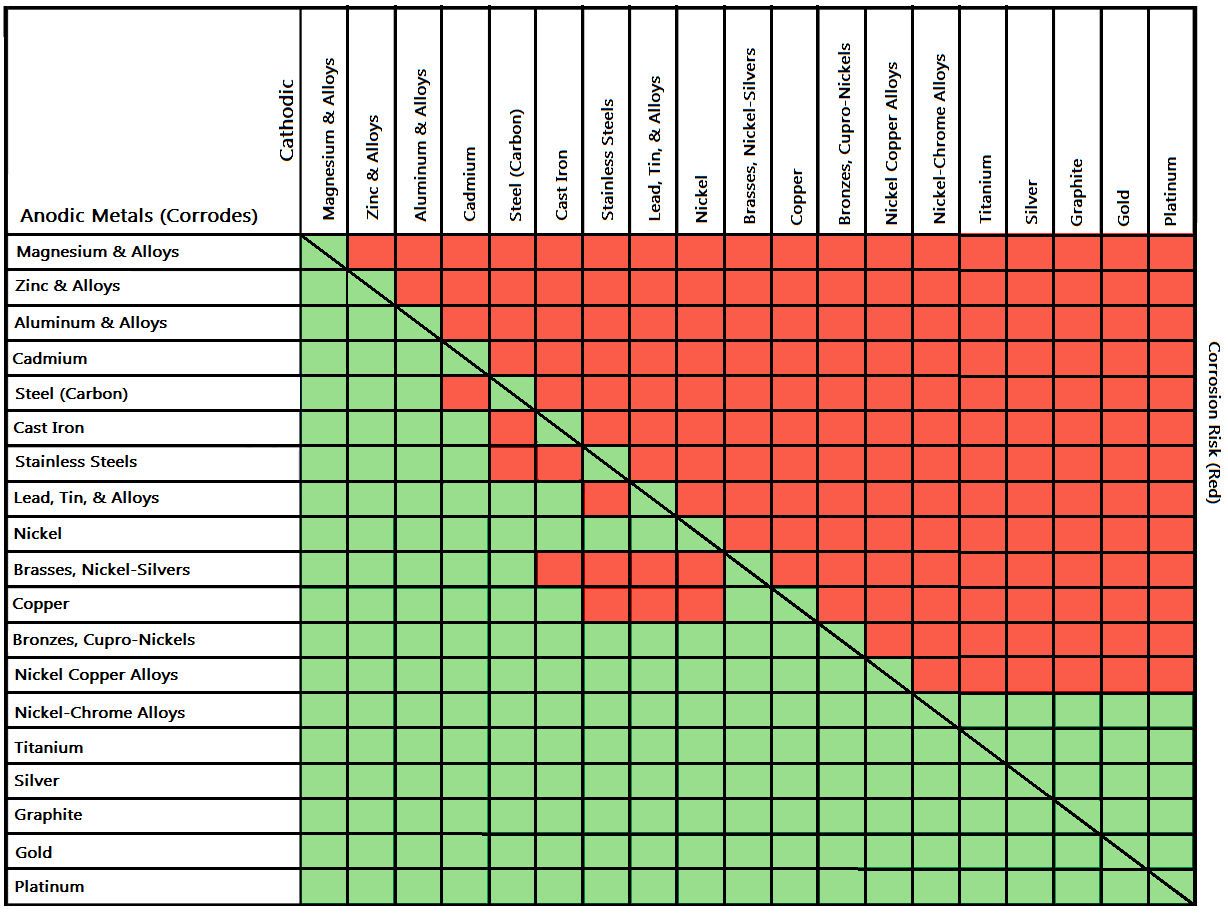
Galvanic Reaction Chart - The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. Web to simplify choosing two compatible metals, a galvanic reaction chart, such as the one below, may be used: The closer a metal or an alloy is in the galvanic series, the less are the effects of galvanic corrosion compared to those metals far apart. Maximum recommended voltage difference is 0,2v: Different alloys of the same metal, such as different types of steel or aluminum, will have different abilities to withstand galvanic corrosion. Web what is galvanic corrosion: Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web galvanic reaction chart below is a galvanic reaction chart for dissimilar metals. Web the galvanic cell is similar to a battery, consisting of two dissimilar metals immersed in an electrolyte solution. Web however,. In this article, we will discuss what is galvanic corrosion, its applications, and how to prevent it using a galvanic corrosion chart? The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. Web however, you can completely avoid galvanic corrosion by choosing matching. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web galvanic series chart. Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. Web galvanic. Web to simplify choosing two compatible metals, a galvanic reaction chart, such as the one below, may be used: Web what is galvanic corrosion: Web this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. By knowing the relationships of the metals in the series,. The closer a metal or an alloy is in the galvanic series, the less are the effects of galvanic corrosion compared to those metals far apart. Web galvanic reaction chart below is a galvanic reaction chart for dissimilar metals. The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two. Web what is galvanic corrosion: Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: There is a link to download the pdf with this information and chart below the table. Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in. The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. Web galvanic reaction chart below. Web galvanic series chart. Maximum recommended voltage difference is 0,2v: The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. By knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic corrosion.. Different alloys of the same metal, such as different types of steel or aluminum, will have different abilities to withstand galvanic corrosion. Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Use this chart below to better understand what metals will work best together. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web the galvanic cell is similar to a battery, consisting of two dissimilar metals immersed in an electrolyte solution. Different alloys of the same metal, such as different types of steel or aluminum, will have different abilities to withstand galvanic corrosion. Maximum recommended voltage difference is 0,2v: Web metals that are further from each other have the highest rate of corrosion when combined. Web what is galvanic corrosion: Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Web to simplify choosing two compatible metals, a galvanic reaction chart, such as the one below, may be used: Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. The closer a metal or an alloy is in the galvanic series, the less are the effects of galvanic corrosion compared to those metals far apart. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: There is a link to download the pdf with this information and chart below the table. An electrochemical reaction occurs involving a transfer of electrons from one metal to the other (electric current) when the two metals are connected by an external, conductive path. In this article, we will discuss what is galvanic corrosion, its applications, and how to prevent it using a galvanic corrosion chart?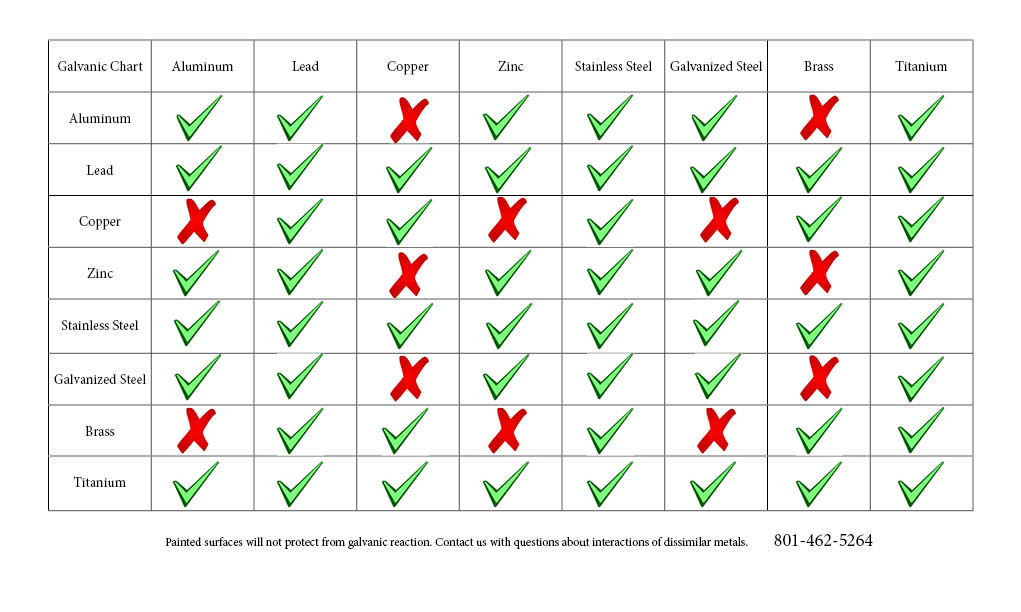
Attaching Stainless Steel to Aluminum DIY Home Improvement Forum

Separating Galvanic Metals JLC Online

Galvanic Reaction Chart All Points Fasteners
Galvanic Potential Chart For Metals My XXX Hot Girl
Dissimilar joining of Al with steel? r/Welding

Stainless Steel Galvanic Corrosion Chart
Galvanic Corrosion SSINA
Galvanic Corrosion Common Questions Answered

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot
Instrumentation Tubing and Their Connections 1.0 Introduction to
Galvanic Series / Galvanic Table.
By Knowing The Relationships Of The Metals In The Series, Galvanic Compatibility Can Be Determined, Preventing The Possible Harmful Effects Of Galvanic Corrosion.
Web This Chart Is Designed To Assist In Broadly Assessing The Risk Of Galvanic Corrosion Associated With A Given Metal Coming Into Contact With Another Metal.
Web Galvanic Reaction Chart Below Is A Galvanic Reaction Chart For Dissimilar Metals.
Related Post: