Molecular Geometry Chart With Hybridization
Molecular Geometry Chart With Hybridization - Sp hybridization can explain the linear structure in molecules. To draw the lewis structure of co, it is vital to know the total number of valence electrons of the molecule. 2f2 + 2naoh ——> of2 +. Web to determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions. Sp2, trigonal planar, trigonal planar. You don't have any studylists yet. You haven't viewed any documents yet. Due to this reason, it is essential to study its lewis structure. Dispose of naphthalene in the labeled beaker in hood 1. Want to join the conversation? Molecular geometry (10.1) dipole moments (10.2) valence bond theory (10.3) hybridization of atomic orbitals (10.4) hybridization in molecules containing double and triple bonds (10.5) molecular orbital theory (10.6) chemical bonding ii. Orbitals are combined in order to spread out electrons. Vsepr theory and molecular geometry. A hybrid or a mix of atomic orbitals. Two electron groups are involved resulting in. Due to this reason, it is essential to study its lewis structure. Molecular geometry • lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. With five electron groups, the lowest energy arrangement is a trigonal bipyramid, as shown in figure 6.3.5. Two electron groups are involved resulting. The angle between the orbitals is 180°. Web hybridization of atomic orbitals. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. There are five groups around sulfur, four bonding pairs and one lone pair. With five electron groups, the lowest. 5 min read • december 28, 2022. Web with an expanded valence, this species is an exception to the octet rule and the hybridization of orbitals on the s atom is sp3d. Web the five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. This document shows the molecular geometries and. Web summary vsepr and hybridization. Hydrogen peroxide comprises two hydrogen atoms and two oxygen atoms; This document shows the molecular geometries and. Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). With five electron groups, the lowest energy arrangement is a trigonal bipyramid, as shown in figure 6.3.5. Web. Valence shell electron pair repulsion (vsepr) lewis structures. Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). Web shapes of molecules and hybridization a. To draw the lewis structure of co, it is vital to know the total number of valence electrons of the. Web determining molecular shape (vsepr) hybridization *molecular orbital theory (bond order, diamagnetism, paramagnetism) coordination compounds and their biological importance naming shape, structure, coordination number, ligands biological examples industrial examples *stereochemistry *crystal field theory *molecular. Hybridisation and geometry of molecules play a vital role in their reactivity. Can determine properties such as geometry, bond orders, bond lengths, and dipoles for molecules.. The front lobes face away from each other and form a straight line leaving a 180° angle between the two orbitals. Hydrogen peroxide comprises two hydrogen atoms and two oxygen atoms; To draw the lewis structure of co, it is vital to know the total number of valence electrons of the molecule. Hence we will need to know the valence. Hydrogen peroxide comprises two hydrogen atoms and two oxygen atoms; The equation for the preparation of oxygen difluoride: Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. A hybrid or a mix of atomic orbitals. To draw the lewis structure of co, it is vital to know the total number of valence electrons of the molecule. Web the five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. The equation for the preparation of oxygen difluoride: The angle between adjacent bonds of an. You haven't viewed any documents yet. Web hybridization of atomic orbitals. If all the bonds are in place the shape is also trigonal planar. You don't have any books yet. Hence we will need to know the valence electrons for both atoms to get the total valence electrons for h2o2. Sp hybridization can explain the linear structure in molecules. This document shows the molecular geometries and. The front lobes face away from each other and form a straight line leaving a 180° angle between the two orbitals. Hybridisation and geometry of molecules play a vital role in their reactivity. Molecular orbital = overlap of two atomic orbitals from different atoms. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Orbitals are combined in order to spread out electrons. Dispose of solvents in the labeled beaker in hood 1. Molecular geometry (10.1) dipole moments (10.2) valence bond theory (10.3) hybridization of atomic orbitals (10.4) hybridization in molecules containing double and triple bonds (10.5) molecular orbital theory (10.6) chemical bonding ii. Electron domains, vsepr and determining molecular geometries. Web let us discuss the basic concepts of ethane such as its lewis structure, polarity, hybridization of carbon atom in ethane, and its molecular orbital (mo) diagram to understand its chemical bonding in terms of molecular orbitals.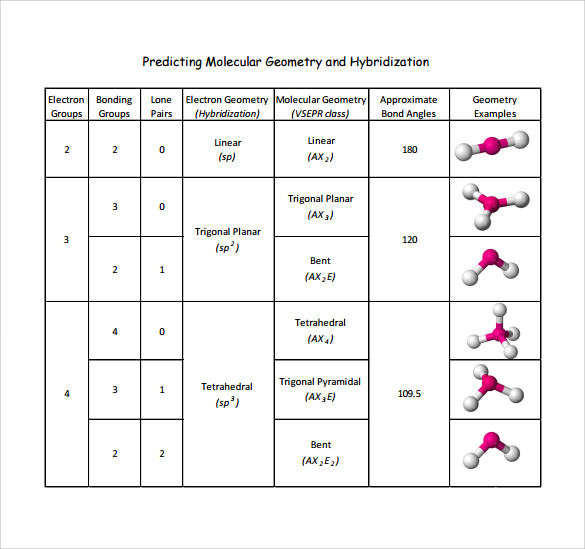
Molecular geometry and VSEPR Molecular geometry, Chemistry worksheets

XeO2F2 Lewis Structure, Geometry, Hybridization, and Polarity

Compound molecular geometry table kowerncure

Molecular Geometry and Hybridization Diagram Quizlet

Hybridization of Orbitals Chemistry Topics Enseñanza de química
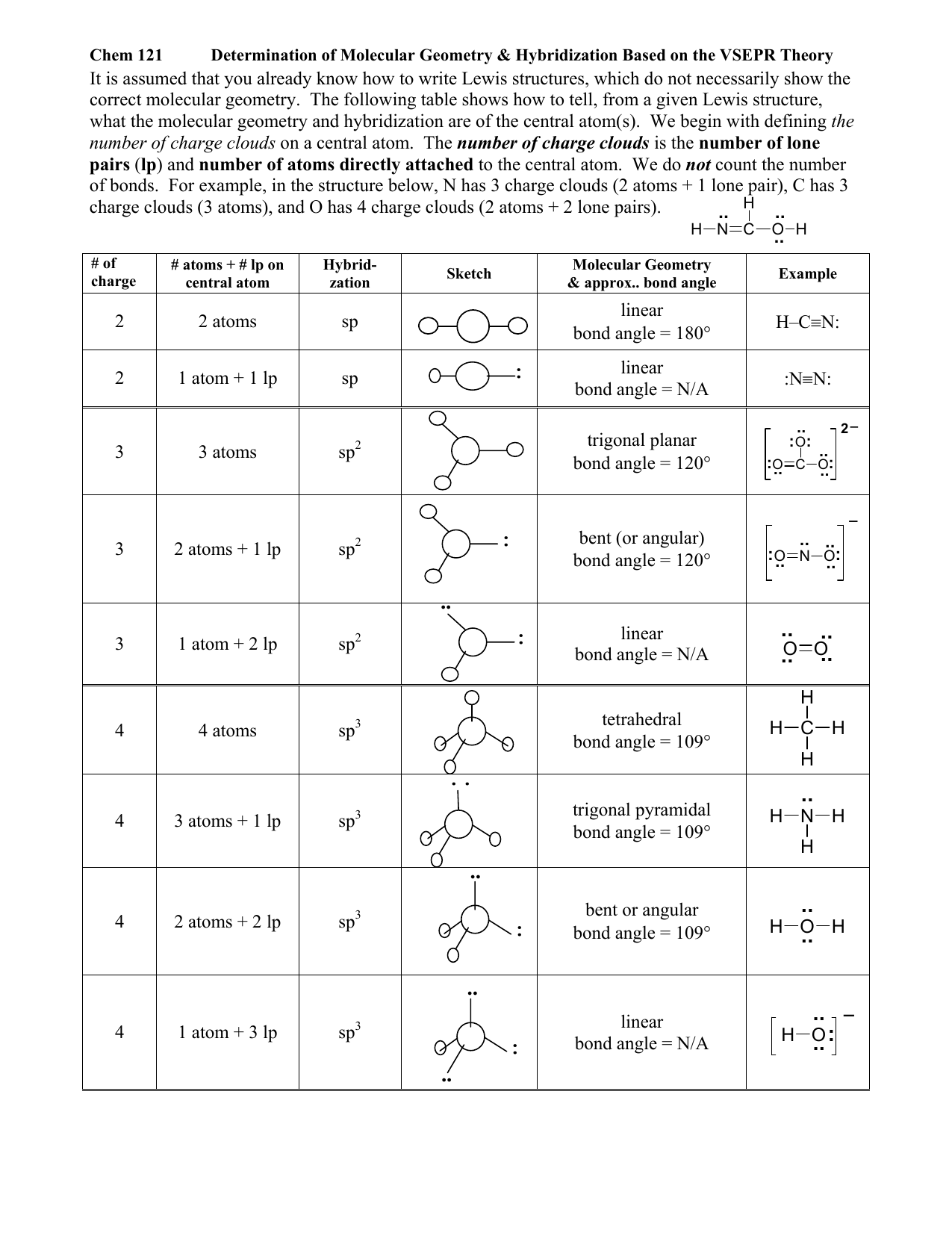
Hybridization Chart
What is the correct order of bond polarity of the bonds F F H F and O F
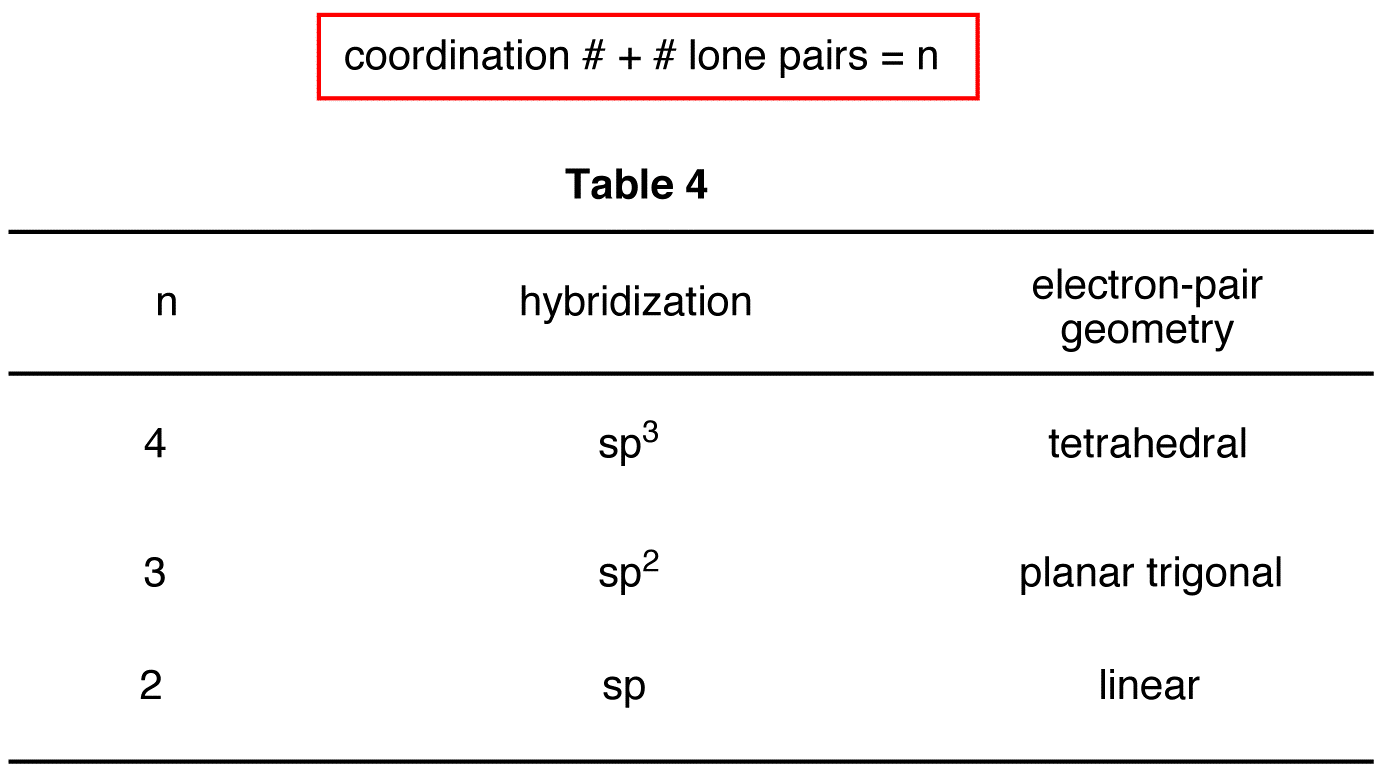
Hybridization And Geometry Chart

Molecular Geometry Chart With Hybridization carthopde

Hybridization Chart
To Draw The Lewis Structure Of Co, It Is Vital To Know The Total Number Of Valence Electrons Of The Molecule.
5 Min Read • December 28, 2022.
Web For Sp2 Hybridized Central Atoms The Only Possible Molecular Geometry Is Trigonal Planar.
You Don't Have Any Courses Yet.
Related Post: