Pka Chart Amino Acids
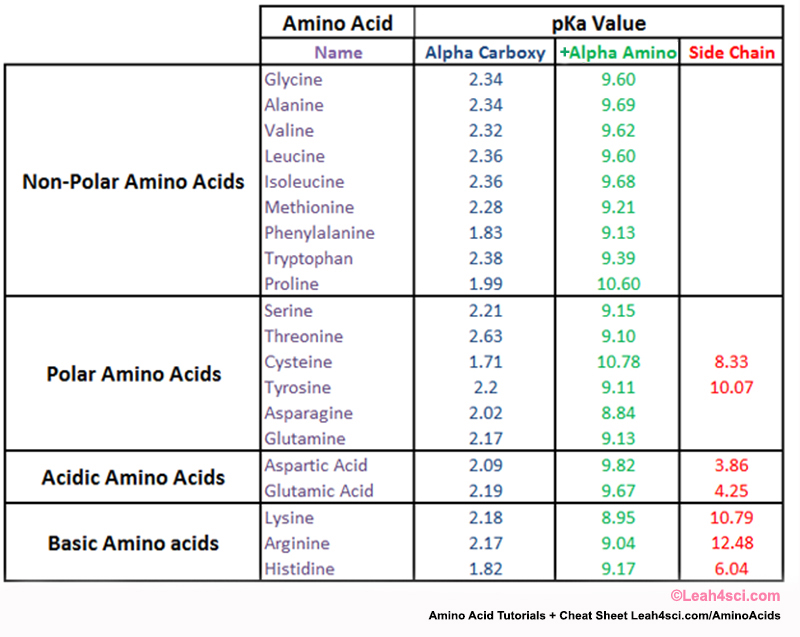
Pka Chart Amino Acids - Created by tracy kim kovach. Amino acids, proteins, and enzymes. Properties of common amino acids. The pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. The side chains of acid and basic amino acids, and some polar amino acids can also be titrated: That is a daunting task for 20 amino acids. What is an amino acid chain? Properties of common amino acids. Pka values in dmso by functional groups; The isoelectric points range from 5.5 to 6.2. What is an amino acid chain? The isoelectric points range from 5.5 to 6.2. 13 α,carboxyl of free amino acid: Inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4, 8 pyrazine 26 aliphatic 4, 8 aromatic 7, 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10, 11 quinoxaline 27 amino acids 12 special nitrogen compounds. 3 n,terminal amino of peptide: Amino acids, proteins, and enzymes. What is an amino acid chain? 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. Web amino acids reference chart. The isoelectric points range from 5.5 to 6.2. The pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. 2 α,amino of free amino acid: Web most biochemistry courses will require you to know the following: 3 pkx is the negative of the logarithm of the dissociation constant for any other. Web amino acids reference chart. Pka values in dmso by functional groups; Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. Web an amino acid is a compound that contains both an amine group (−nh2) ( − nh 2) and a carboxyl group (−cooh) ( − cooh) in the same molecule. Amino acids are. Properties of common amino acids. Web an amino acid is a compound that contains both an amine group (−nh2) ( − nh 2) and a carboxyl group (−cooh) ( − cooh) in the same molecule. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. The r group side chains may be either nonpolar, polar and. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. Web the proteinogenic amino acids are amphoteric and have two or three pk values, depending on their side chains. Web amino acids reference chart. Want to join. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. Titration curves show the neutralization of these acids by added base, and the. Web pka data compiled by r. Amino acids are the building blocks that make up all proteins, polypeptides and peptides. Pka values in gas phase; While any number of amino acids can possibly be imagined, biochemists generally reserve the term for a group of 20 amino acids which are formed and used by living organisms. The pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Ripin) pka values in water; 2 α,amino of free amino. Web pka data compiled by r. The side chains of acid and basic amino acids, and some polar amino acids can also be titrated: Web amino acids reference chart. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. These numbers are taken from one of many scales that describe the hydrophobicity of. Web an amino acid is a compound that contains both an amine group (−nh2) ( − nh 2) and a carboxyl group (−cooh) ( − cooh) in the same molecule. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. This. What is an amino acid chain? Titration curves show the neutralization of these acids by added base, and the. This online tool may be cited as follows. Properties of common amino acids. 9.5 c,terminal carboxyl of peptide: Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. This video will show you how! Want to join the conversation? Amino acids are the building blocks that make up all proteins, polypeptides and peptides. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. Web the proteinogenic amino acids are amphoteric and have two or three pk values, depending on their side chains. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4, 8 pyrazine 26 aliphatic 4, 8 aromatic 7, 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10, 11 quinoxaline 27 amino acids 12 special nitrogen compounds 28 peptides 13 hydroxylamines 28. While any number of amino acids can possibly be imagined, biochemists generally reserve the term for a group of 20 amino acids which are formed and used by living organisms. 2 α,amino of free amino acid: Web amino acids reference chart.
pKa Values Span 60 Orders Of Magnitude (!) Putting Them In Perspective
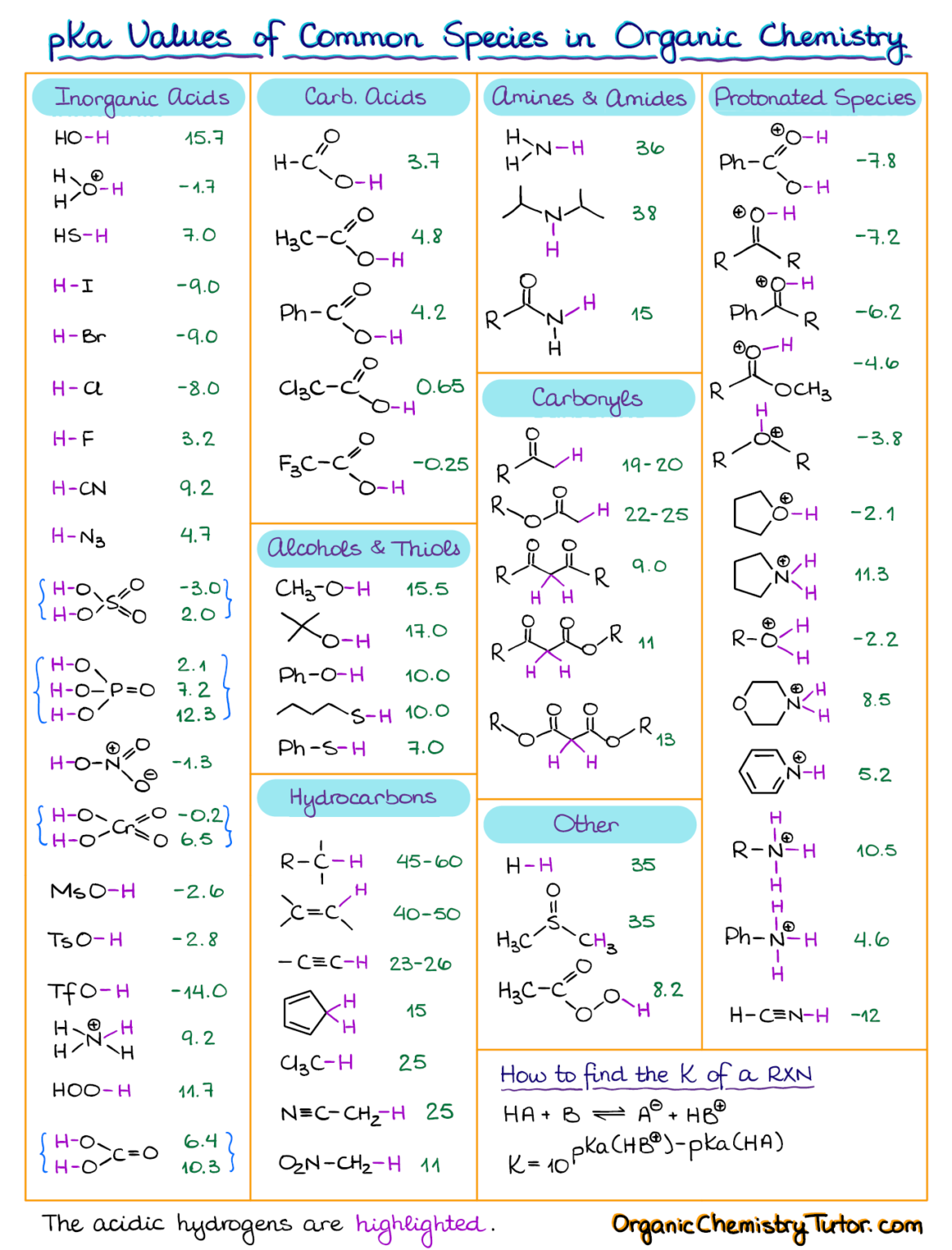
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic

Amino Acid Pka Chart amulette
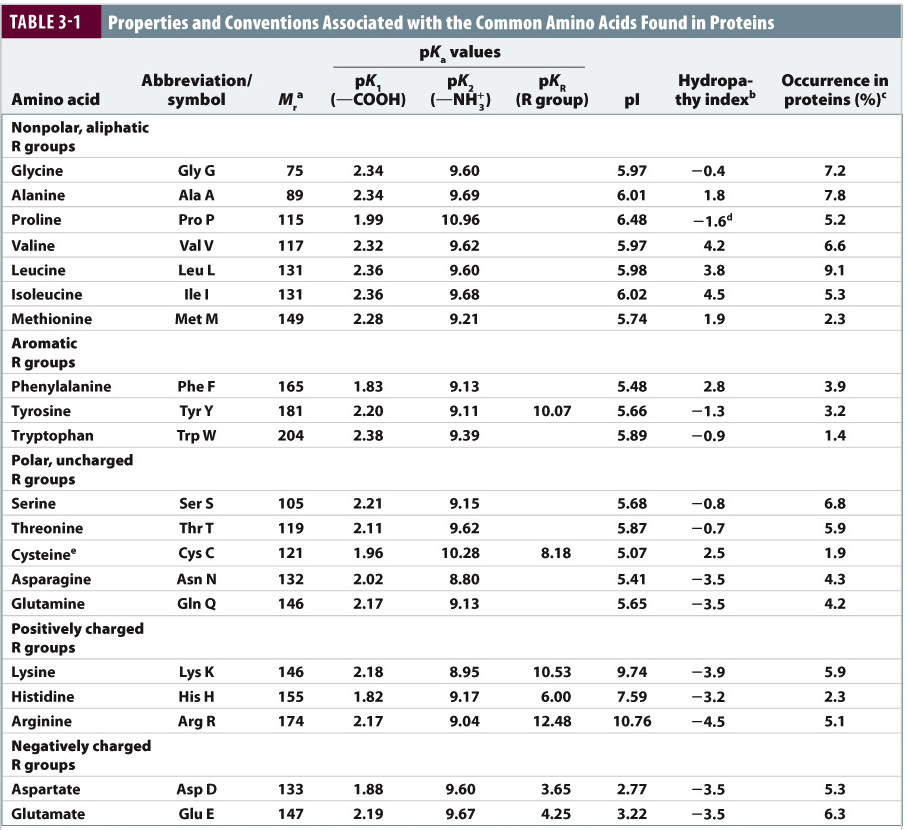
Solved Use the following pka table 1) What is the
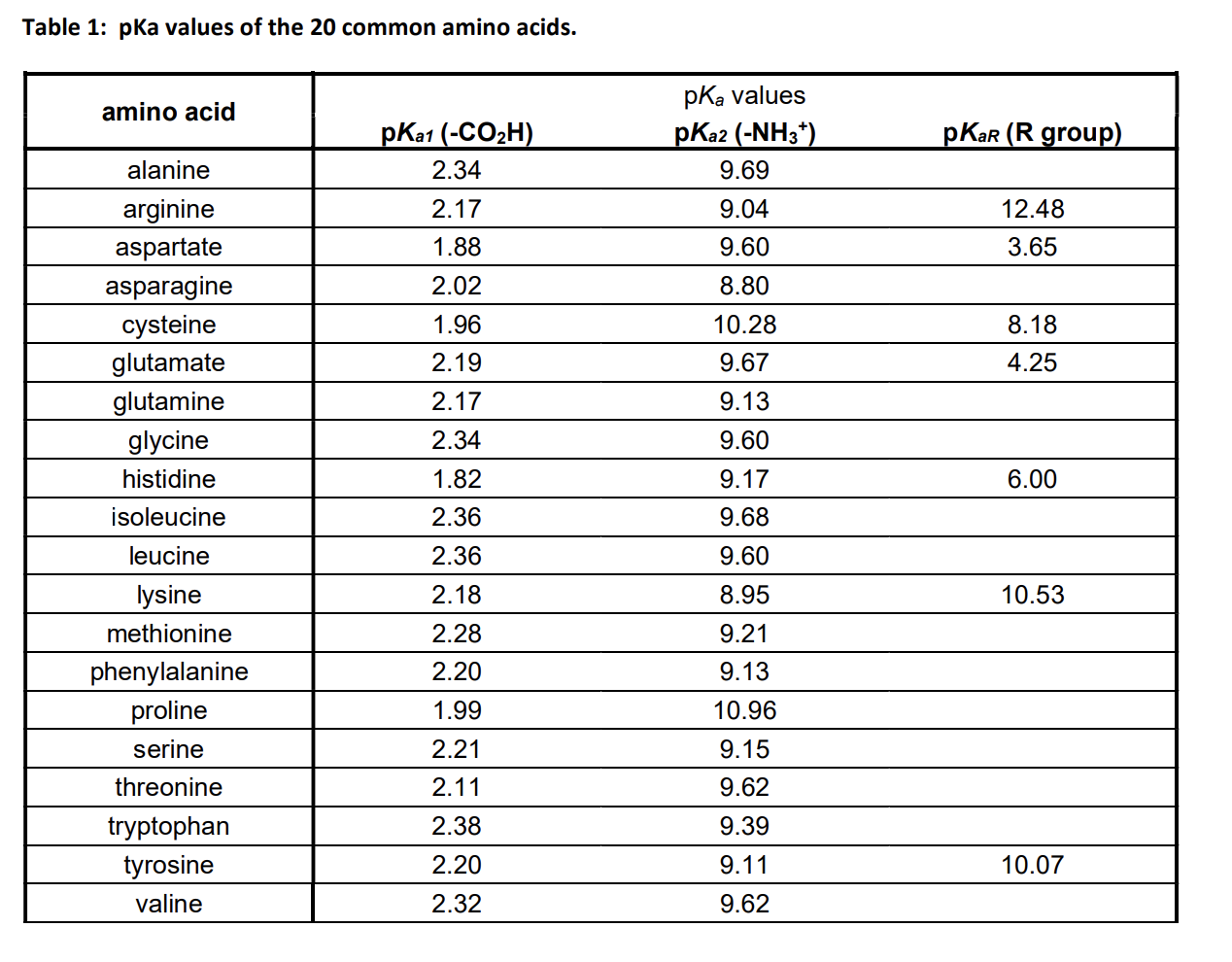
Solved Table 1 pKa values of the 20 common amino acids.
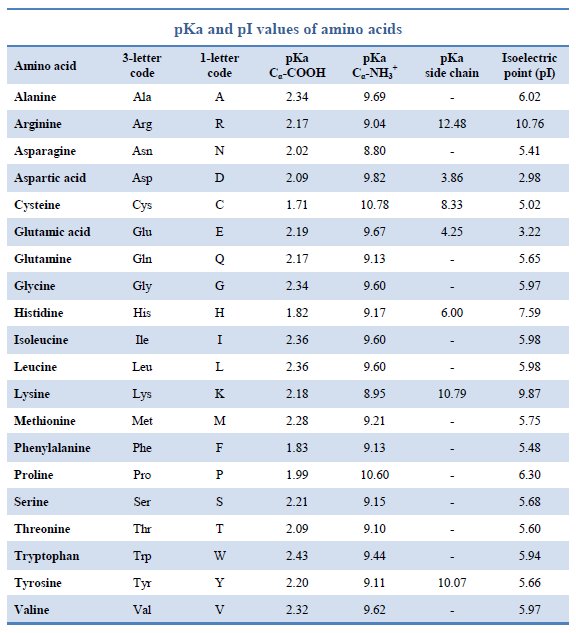
Amino Acids and Proteins Daniel's MCAT Notes

Chapter 2 Protein Structure Chemistry

Amino Acid Pka Table Letter G Decoration Ideas
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial

The pKa in Organic Chemistry Chemistry Steps
What Is An Amino Acid Chain?
Web Pka Data Compiled By R.
Pka Values In Dmso By Functional Groups;
Web Pka Values Compilation (By Dave Evans And D.
Related Post: