Salt Water Freezing Point Chart
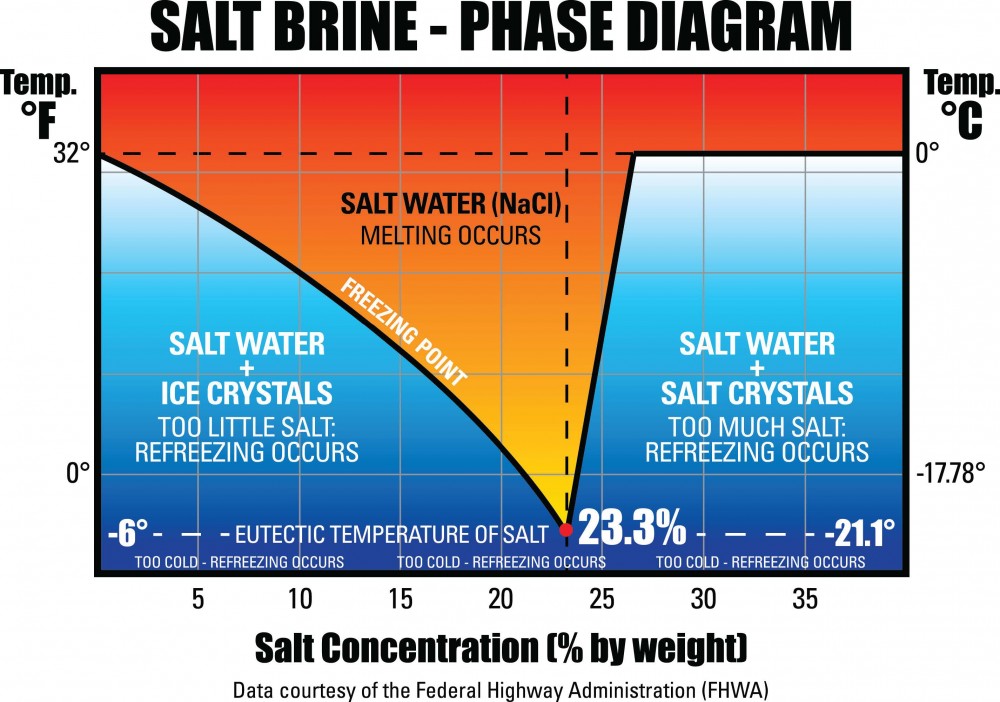
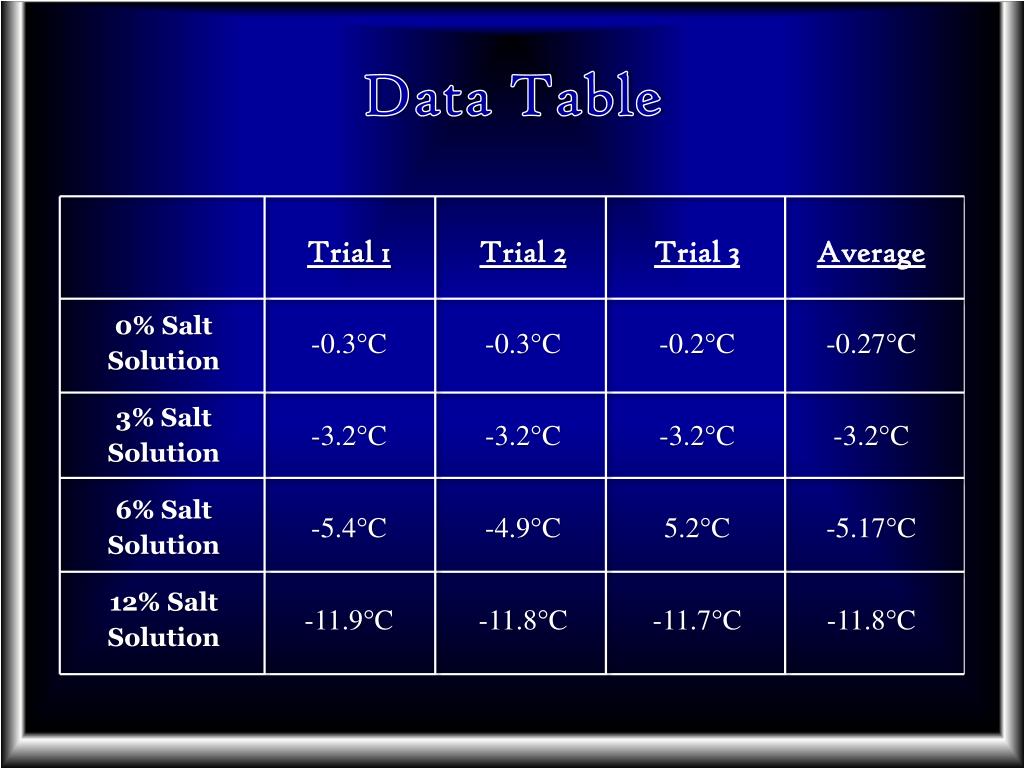
Salt Water Freezing Point Chart - Web this line represents the effect of increasing amounts of salt on the freezing point of water. It’s a clear, tasteless liquid that maintains life and habitat for plants and animals. The more salt there is in the water,. Fill up 5 cups with 100 ml of water each. Web take 14.8 ml (1 tbsp = 3 tsp) of the saline solution and place it in one of the slots in the ice cube tray. The freezing point of salt water is lowered due to the presence of dissolved salt. 30 eutectic point 6f 20 solid 10. Freezing point 60 0 10 20 30 40 50 60 70 80 90 100. As salt is added to this water, its freezing points starts decreasing. Thus, adding 100 grams of salt to 1 kilogram of water would decrease the freezing point to. Test the effect of table salt (sodium chloride) on the freezing point of water. Web if you look it up you'll find that at around 23% salt brine has it's lowest freeze point. It's something like the ratio of pure radiator fluid to water. As it freezes, it expands, creating ice, a vital part of our wintertime activities. Either way. Eventually, all the ice melts, leaving very cold salt water. Salt water freezing point calculator. Web take 14.8 ml (1 tbsp = 3 tsp) of the saline solution and place it in one of the slots in the ice cube tray. Freezing point 60 0 10 20 30 40 50 60 70 80 90 100. Web this line represents the. The na and cl dissociate right away when dissolved, and so for a 0.5 molal solution of salt, there is a. Nacl, wt% freezing point (°c) freezing point (°f) density (g/cm 3) refractive index at 589 nm viscosity (cp ) 0: Eventually, all the ice melts, leaving very cold salt water. Let's say you have a salt water sample with. Most people who produce brine make it in quanities and store it outside. Water is one of the essential substances for life. The freezing point of salt water is lowered due to the presence of dissolved salt. Test the effect of table salt (sodium chloride) on the freezing point of water. It’s a clear, tasteless liquid that maintains life and. Explain it to a child. 30 eutectic point 6f 20 solid 10. It's something like the ratio of pure radiator fluid to water. How do you calculate the freezing point of salt water? Web this line represents the effect of increasing amounts of salt on the freezing point of water. Web freezing point, density, specific heat and dynamic viscosity of sodium chloride and water coolant. Repeat for x equal to 0 ml, 2.5 ml (1/2 tsp), 4.9 ml (1 tsp), 7.4 ml (1.5 tsp), and so on, all the way up to the saturation point of salt in water. 30 eutectic point 6f 20 solid 10. Web in case of. Web for saltwater that’s as saturated as it can possibly get (i.e. Web freezing points for water with freezing mixtures based on salt and ice: Web now, you can use the freezing point depression equation to find the new freezing point of the solution. Web in case of sea water, the average salt content is 35 parts per thousand, and. Web here δt is the change in freezing point in kelvins (celcius will work too since they have the same scale). Web take 14.8 ml (1 tbsp = 3 tsp) of the saline solution and place it in one of the slots in the ice cube tray. Web if you look it up you'll find that at around 23% salt. Web freezing points for water with freezing mixtures based on salt and ice: The more salt there is in the water,. Most people who produce brine make it in quanities and store it outside. Web now, you can use the freezing point depression equation to find the new freezing point of the solution. Fresh water freezes at 32 degrees fahrenheit. Web for saltwater that’s as saturated as it can possibly get (i.e. 30 eutectic point 6f 20 solid 10. The freezing point of salt water is lowered due to the presence of dissolved salt. Explain it to a child. How do you calculate the freezing point of salt water? Web now, you can use the freezing point depression equation to find the new freezing point of the solution. Fluid freezing or melting point (k) acetic acid: However, salt can change the normal freezing process of water by lowering its freezing point. Web take 14.8 ml (1 tbsp = 3 tsp) of the saline solution and place it in one of the slots in the ice cube tray. Web this line represents the effect of increasing amounts of salt on the freezing point of water. In water in which the concentration of salt. Explain it to a child. The freezing point of salt water is lowered due to the presence of dissolved salt. Fill up 5 cups with 100 ml of water each. Thus, adding 100 grams of salt to 1 kilogram of water would decrease the freezing point to. Web freezing points for water with freezing mixtures based on salt and ice: It’s a clear, tasteless liquid that maintains life and habitat for plants and animals. How do you calculate the freezing point of salt water? The freezing point of salt water depends on the concentration of salt. As salt is added to this water, its freezing points starts decreasing. Water is one of the essential substances for life.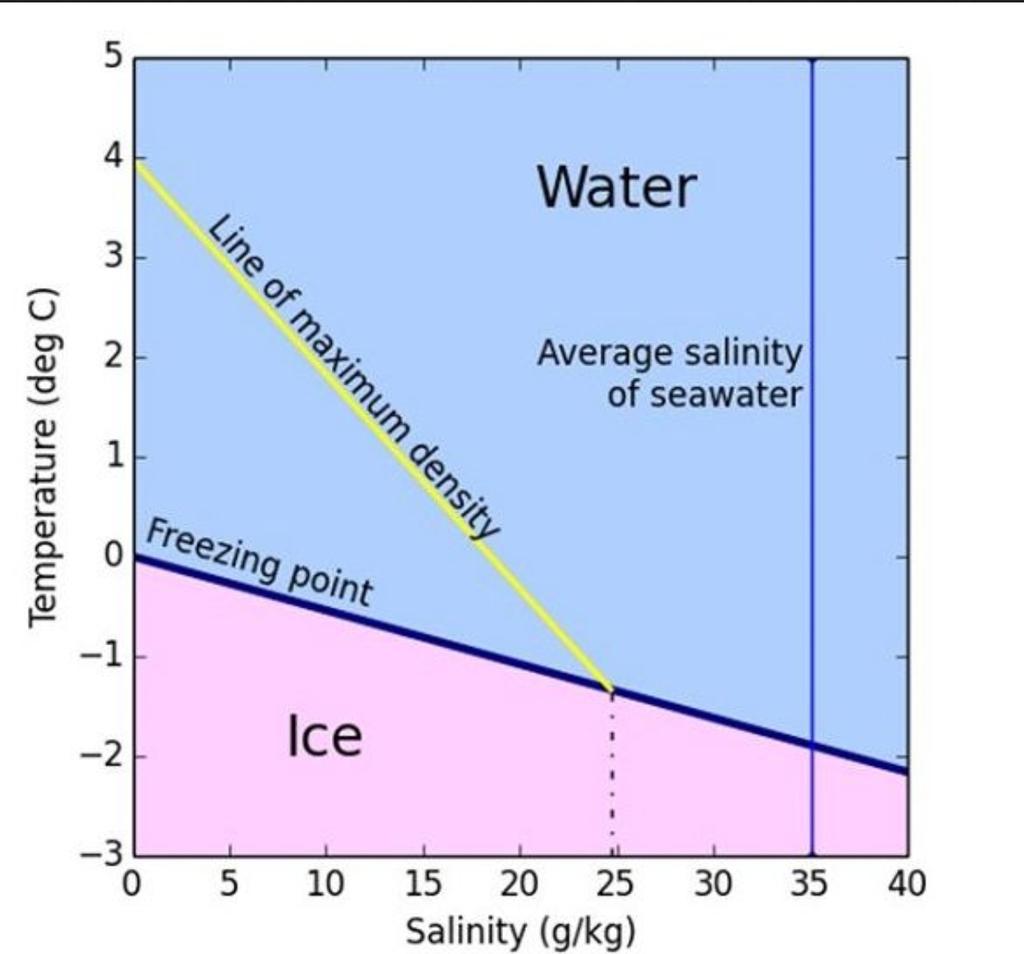
tmallard on Twitter "LeonSimons8 Quandary The Pacific & Atlantic
Minimizing the impact of ice The Municipal
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation
Build ice towers with bottled water and ice

Physics 111 Fundamental Physics I The Physics of Salting Roads

Freezing temperatures of salt brine mixtures Download Scientific Diagram
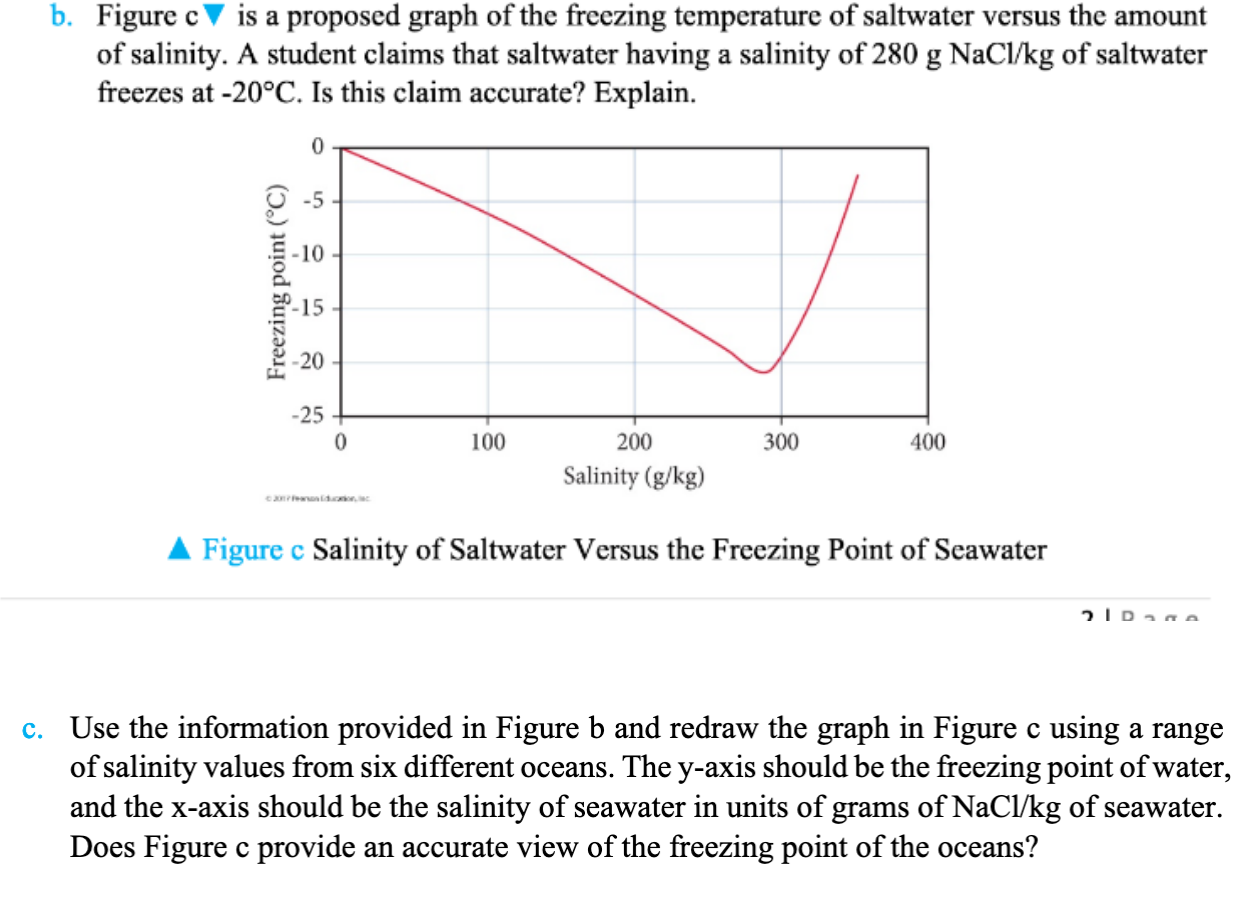
b. Figure c is a proposed graph of the freezing

winter What is the safest and most effective additive to keep
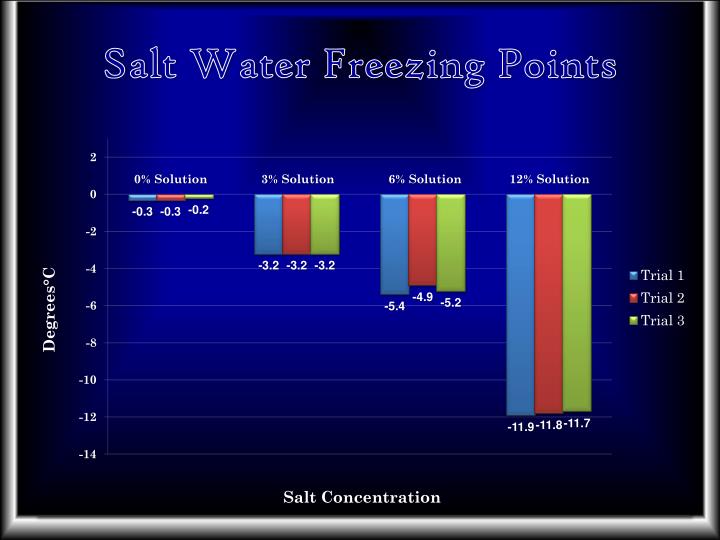
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation
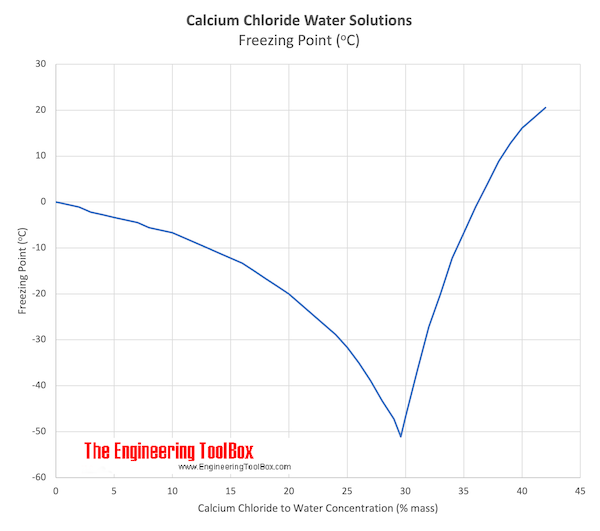
Calcium Chloride and Water
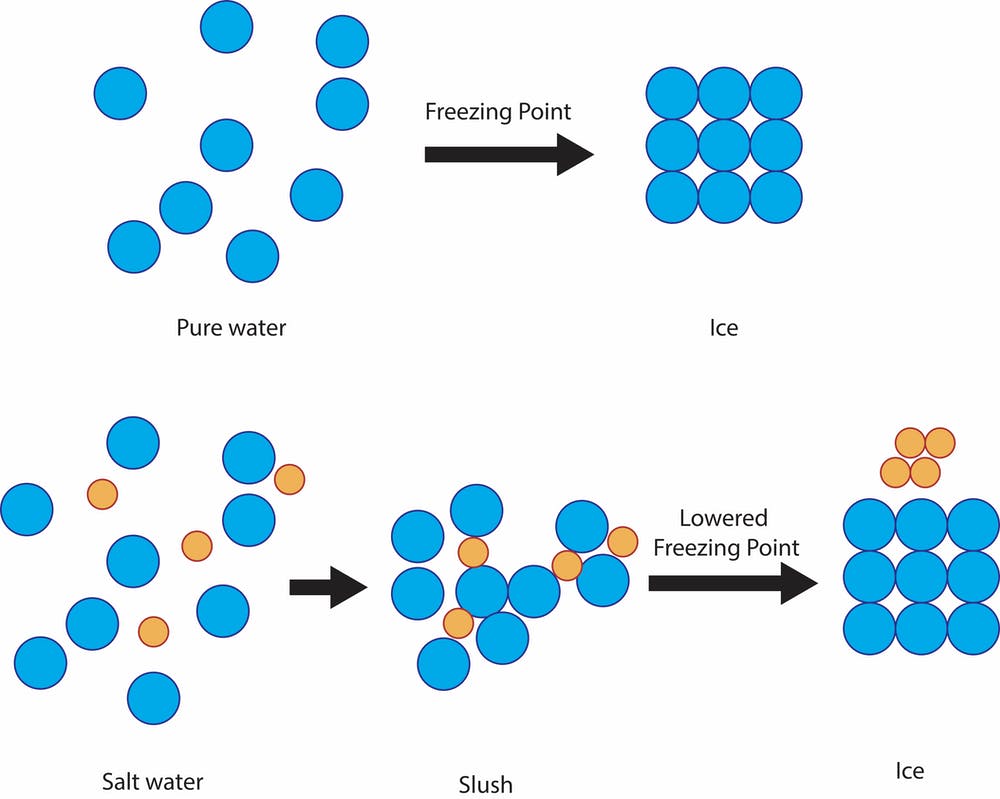
As The Water Starts To Freeze, The Salt Gets Left In The Liquid.
Web In Case Of Sea Water, The Average Salt Content Is 35 Parts Per Thousand, And Therefore, Its Freezing Point Is −1.8°C (−28.9°F).
Of Salt Brine 50 Mixtures Liquid.
To Calculate The Freezing Point Of The Salt Water, Use The Following Formula:
Related Post: