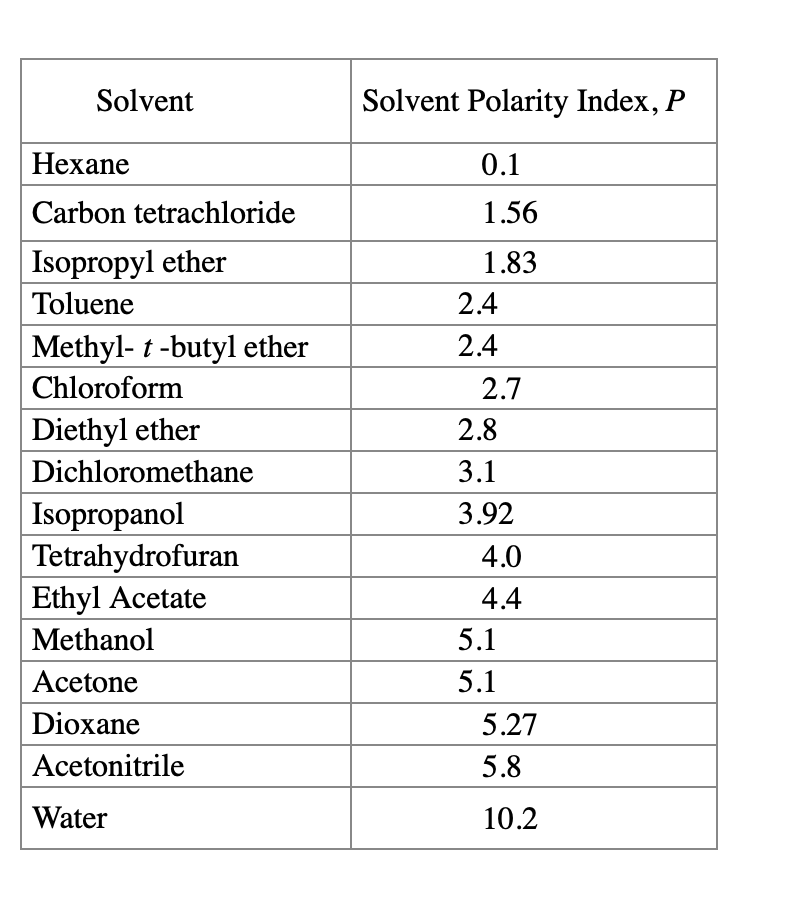
Solvent Polarity Chart
Solvent Polarity Chart - Some solvent pairs commonly used in crystallization are shown on p. Gsk green chemistry solvent selection guide. We learned that salt is made up of cations and anions. Polarity indexes of solvents which are commonly used for sec analysis is shown below. Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. The following video explains why water (a solvent) is polar. Web in order to understand why salts dissolve in water, we have to first understand solvent polarity. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. Due to the polar characteristic of water, this explains why salt dissolves! Density at 20°c (g/ml) acetone : Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. The following video explains why water (a solvent) is polar. Gsk green chemistry solvent selection guide. Some solvent pairs commonly used in crystallization are shown on p. Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive. Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive index : We learned that salt is made up of cations and anions. Web in order to understand why salts dissolve in water, we have to first understand solvent polarity. The following video explains why water (a solvent) is polar. Due to the polar characteristic of water, this explains why. The partially negatively charged oxygen end (of water. Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. Gsk green chemistry solvent selection guide. Solvents used in organic chemistry are characterized by their physical characteristics. Density at 20°c (g/ml) acetone : Some solvent pairs commonly used in crystallization are shown on p. Web water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethyl ether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether hex. The partially negatively charged oxygen end (of water. Density at 20°c (g/ml) acetone : Web solvent. Web weighing reactants and reagents. Web solvent solvent group : Due to the polar characteristic of water, this explains why salt dissolves! Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. Web two solvents of different polarities are mixed in order. We learned that salt is made up of cations and anions. Web in order to understand why salts dissolve in water, we have to first understand solvent polarity. Web solvent solvent group : Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. Gsk green chemistry solvent. Density at 20°c (g/ml) acetone : Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive index : Due to the polar characteristic of water, this explains why salt dissolves! Web solvent solvent group : Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. The partially negatively charged oxygen end (of water. Web solvent solvent group : Gsk green chemistry solvent selection guide. Density at 20°c (g/ml) acetone : Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. The partially negatively charged oxygen end (of water. The following video explains why water (a solvent) is polar. Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive index : Solvents used in organic chemistry are characterized by their physical characteristics. Web solvent solvent group : Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive index : The following video explains why water (a solvent) is polar. Web water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethyl ether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether hex. Polarity indexes of solvents which. Web two solvents of different polarities are mixed in order to achieve an intermediate degree of polarity that depends on their relative proportions. Web solvent solvent group : The following video explains why water (a solvent) is polar. Gsk green chemistry solvent selection guide. The partially negatively charged oxygen end (of water. Some solvent pairs commonly used in crystallization are shown on p. Polarity index (p1) boiling point (°c) viscosity 20°c (cp) refractive index : Solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. Polarity indexes of solvents which are commonly used for sec analysis is shown below. Web weighing reactants and reagents. Due to the polar characteristic of water, this explains why salt dissolves!
organic solvent polarity chart Bamil

2 Values for the KamletTaft solvent polarity scale (continued
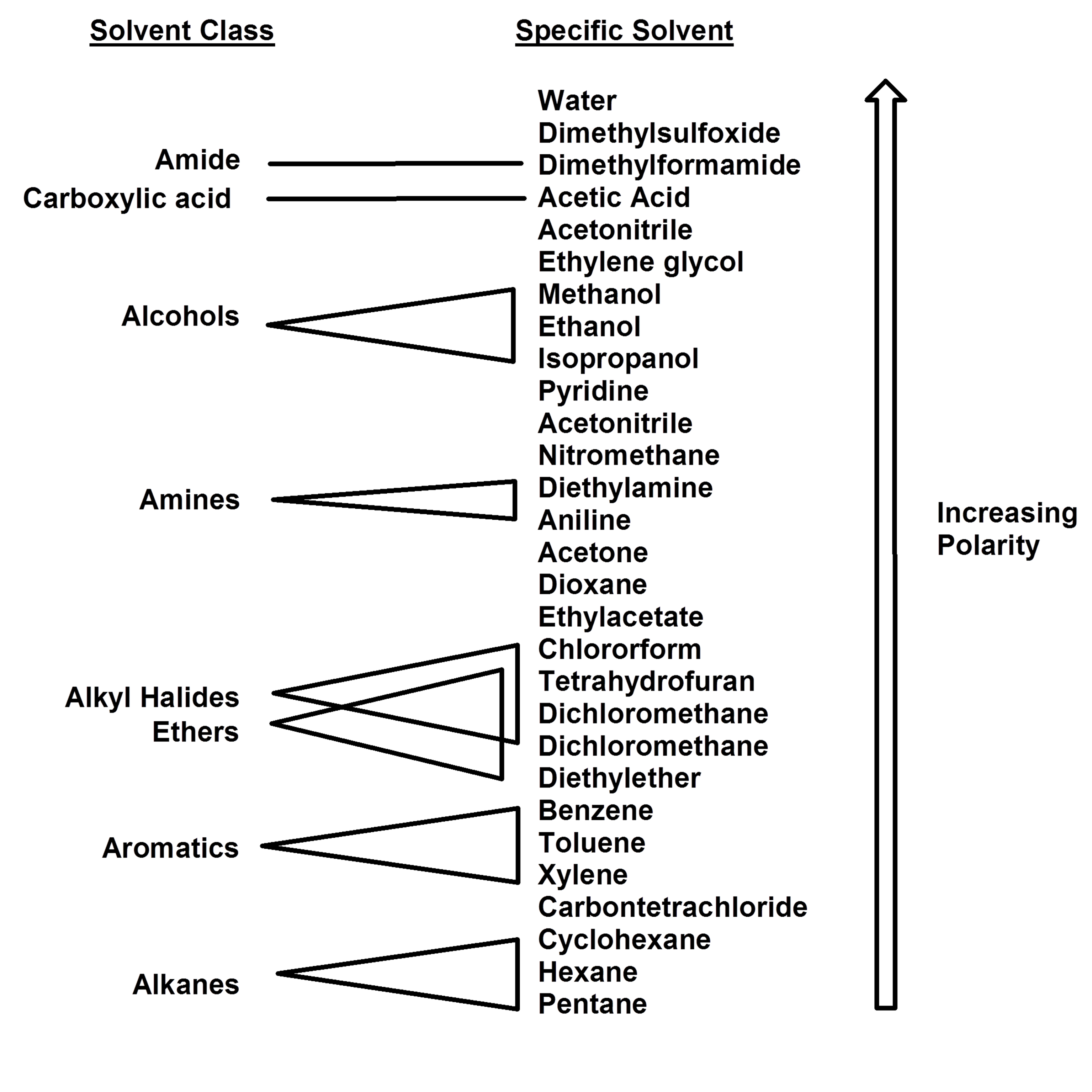
elite Page 3
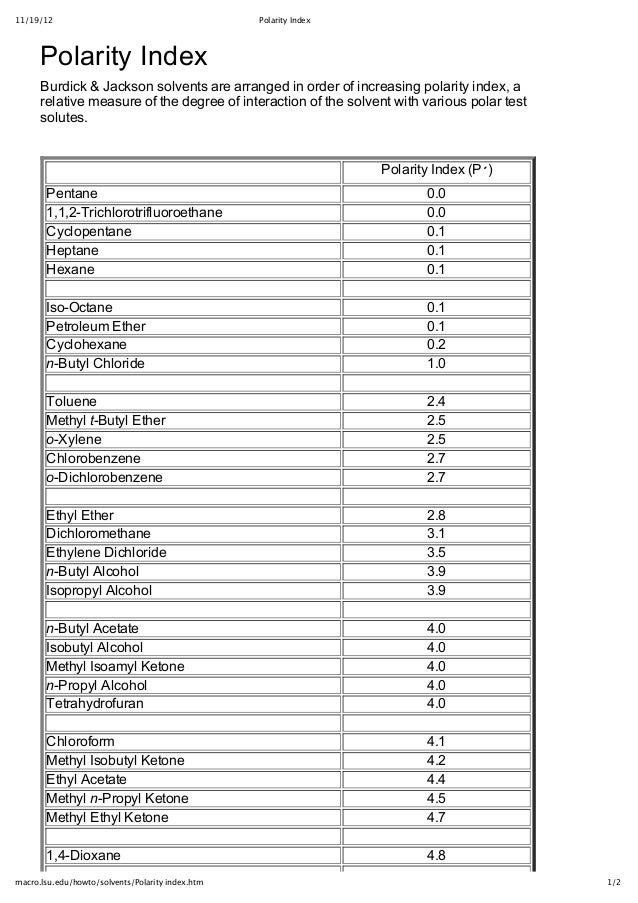
Polarity index
Solved Determine the solvent polarity index for each HPLC
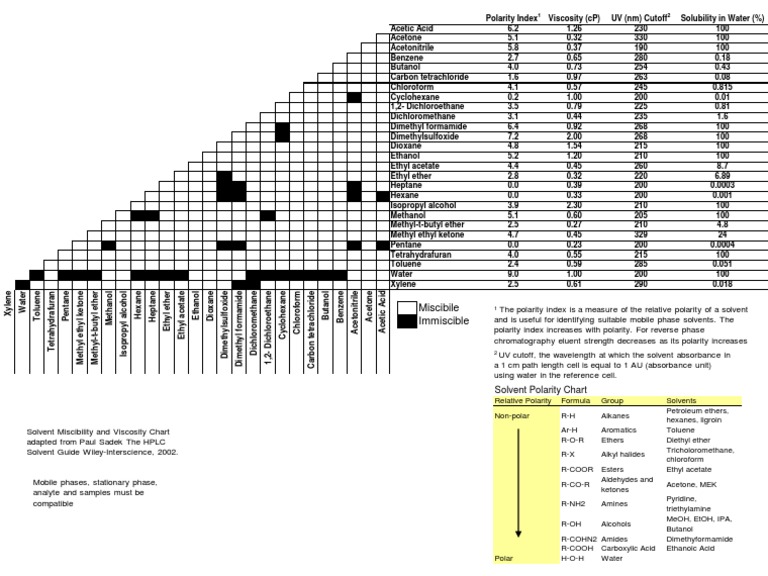
Solvent Miscibility and Polarity Chart
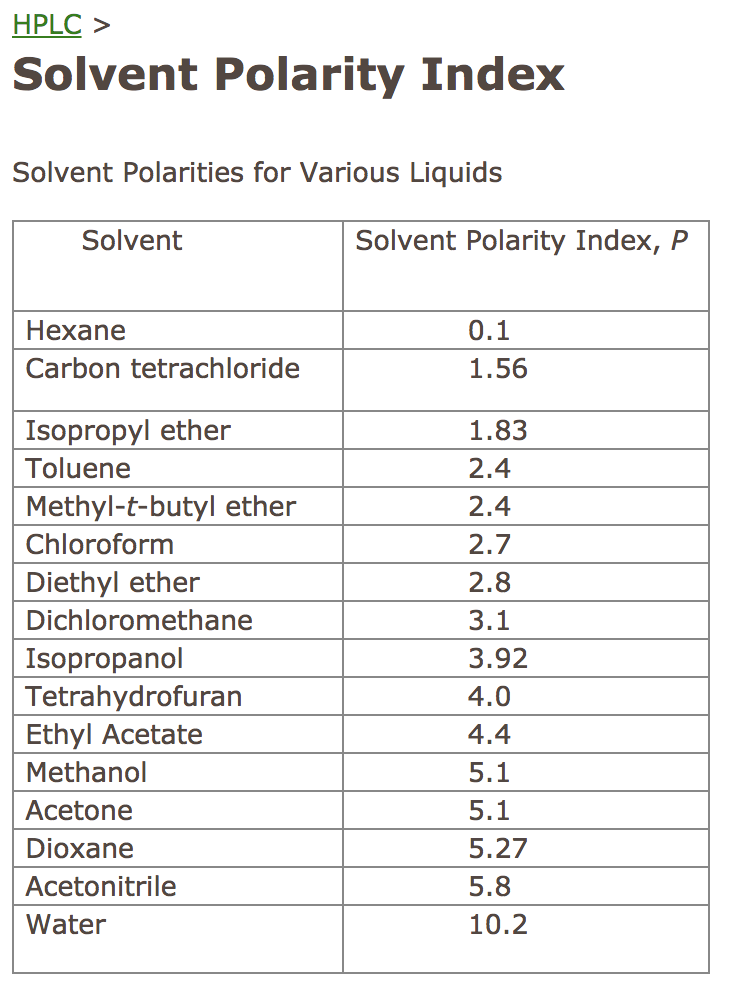
Solved Determine the solvent polarity index for the

Chemistry Solvent Characteristics CTG Clean

Resources An Introduction to Colloidal and Self

Solvents List Solvent Polarity of Some DES Download Table / Without
Density At 20°C (G/Ml) Acetone :
We Learned That Salt Is Made Up Of Cations And Anions.
Web Water Acetic Acid Ethylene Glycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diethylamine Aniline Dimethylsulfoxide Ethyl Acetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethyl Ether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum Ether Hex.
Web In Order To Understand Why Salts Dissolve In Water, We Have To First Understand Solvent Polarity.
Related Post: