Vapor Pressure Chart For Water
Vapor Pressure Chart For Water - A substance with a high vapor pressure is said to be volatile. We also created a water vapor pressure chart ranging from water vapor pressure chart 1°c to 150°c. Conversely, vapor pressure decreases as the temperature decreases. We did the same thing with the kelvin temperature scale, including a 273k to 400k vapor pressure chart. In other words, vapor pressure equals atmospheric pressure at a liquid’s boiling point. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. The pressure scale (the vertical one) is measured in kilopascals (kpa). Selected pressure observations and best track minimum central pressure curve for hurricane. P2 is the vapor pressure at temperature t 2, with si units of atm. Web the vapor pressure of water at 80 °c will be 47.27 kpa (antoine formula) or 46.19 kpa (simple formula). Aldrich, e.w., vapor pressure tables for water, j. The line on the graph shows the boiling temperature for water. Lindsay, w.t., jr., vapor pressure of d 2 o from 106 to 300 ºc, j. The pressure up cancels the pressure down and boiling begins. Selected pressure observations and best track minimum central pressure curve for hurricane. Web water vapor pressure chart. A substance with a high vapor pressure is said to be volatile. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. The “best track” chart of calvin’s path is given in fig. Web the vapor pressure of water at 283 k is 9.2. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa of argon, totaling 102.2 kpa, making the basis for standard atmospheric pressure. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall. Web vapor pressure of water. 1 atmosphere pressure is 101.325 kpa. What is the vapor pressure of water at 343 k? Web the vapor pressure of water at 283 k is 9.2 mmhg, at what temperature is the vapor pressure of water 546 mmhg? The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100. 1 atmosphere pressure is 101.325 kpa. Web so, at 1 atm of pressure, the saturated vapor pressure of water occurs at 100 ° c (212 ° f). We did the same thing with the kelvin temperature scale, including a 273k to 400k vapor pressure chart. Web for example, air at sea level, and saturated with water vapor at 20 °c,. Vapor pressure of water at 25°c and 20°c. To learn more about the details, keep reading! 1 atmosphere pressure is 101.325 kpa. The vapor pressure of a liquid is the point at which equilibrium pressure is reached, in a closed container, between molecules leaving the liquid and going into the gaseous phase and molecules leaving the gaseous phase and entering. Vapor pressure of water at 25°c and 20°c. Web if the liquid is open to the air, then the vapor pressure is seen as a partial pressure along with the other constituents of the air. We also created a water vapor pressure chart ranging from water vapor pressure chart 1°c to 150°c. 1 atmosphere pressure is 101.325 kpa. The line. The line on the graph shows the boiling temperature for water. Vapor pressure of water from 0 °c to 100 °c. Use one of the popular approximations, e.g., antoine formula: What is the vapor pressure of water at 343 k? At 393 k the vapor pressure of water is 1489 mmhg; Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). Web the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. P1 is the vapor pressure at temperature t 1, with si units. Selected pressure observations and best track minimum central pressure curve for hurricane. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Vapor pressure of water from 0 °c to 100 °c. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. The saturation vapor. T1 is the starting temperature, with si units of k. Conversely, vapor pressure decreases as the temperature decreases. At 393 k the vapor pressure of water is 1489 mmhg; 1 atmosphere pressure is 101.325 kpa. P1 is the vapor pressure at temperature t 1, with si units of atm. We also created a water vapor pressure chart ranging from water vapor pressure chart 1°c to 150°c. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). The vapor pressure of a liquid is the point at which equilibrium pressure is reached, in a closed container, between molecules leaving the liquid and going into the gaseous phase and molecules leaving the gaseous phase and entering the liquid phase. Vapor pressure of water from 0 °c to 100 °c. To learn more about the details, keep reading! Web so, at 1 atm of pressure, the saturated vapor pressure of water occurs at 100 ° c (212 ° f). To find the vapor pressure of water: Web if the liquid is open to the air, then the vapor pressure is seen as a partial pressure along with the other constituents of the air. Aldrich, e.w., vapor pressure tables for water, j. Selected pressure observations and best track minimum central pressure curve for hurricane. 2, with the wind and pressure histories shown in figs.
Conservation physics Fundamental microclimate concepts
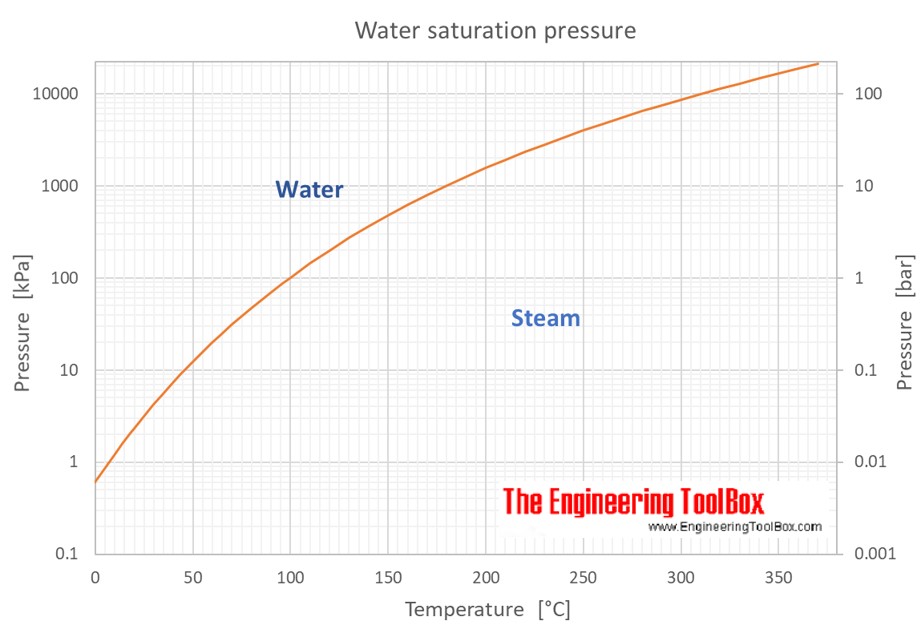
Water Saturation Pressure vs. Temperature
cuvânt înainte hârtie Salut water pressure temperature calculator
![[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A](https://d3i71xaburhd42.cloudfront.net/471011a1b864be3f78720f4d8ba4f21385acd117/6-Table5-1.png)
[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A

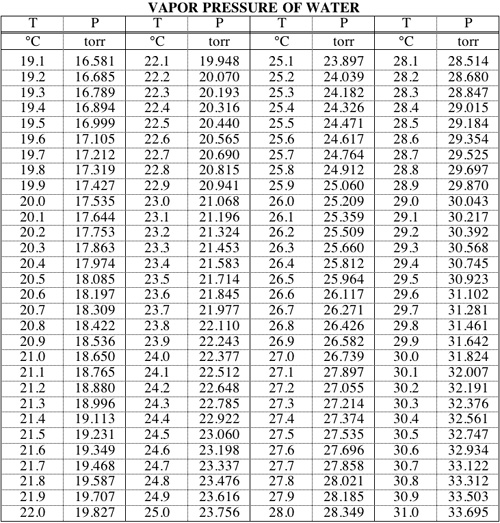
Vapor Pressure Of Water Table Atm
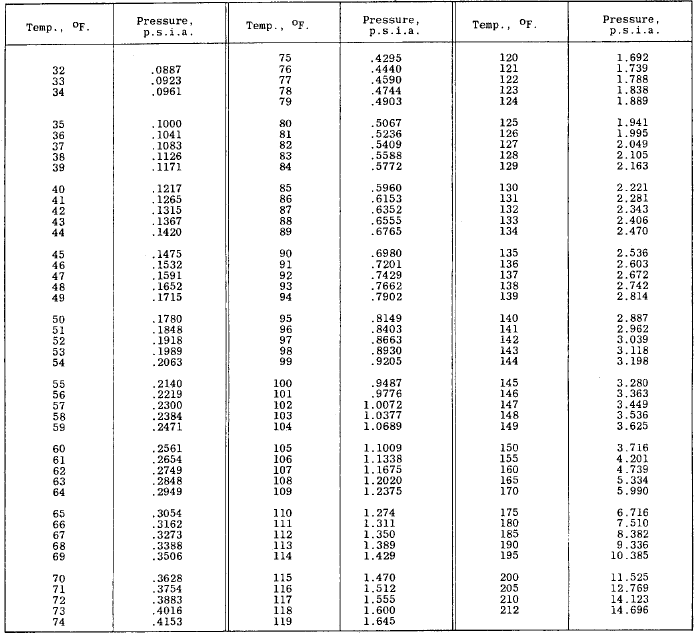
Partial Pressure of Water Vapor in Saturated Air Table Chart
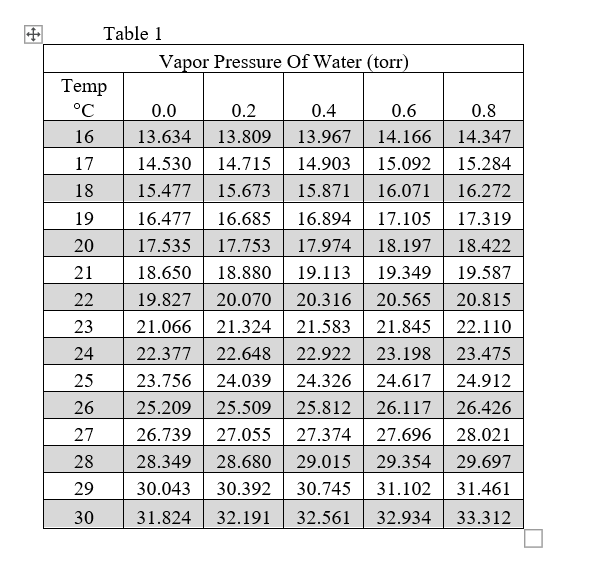
Solved Table 1 Vapor Pressure Of Water (torr) Temp °C 16 17
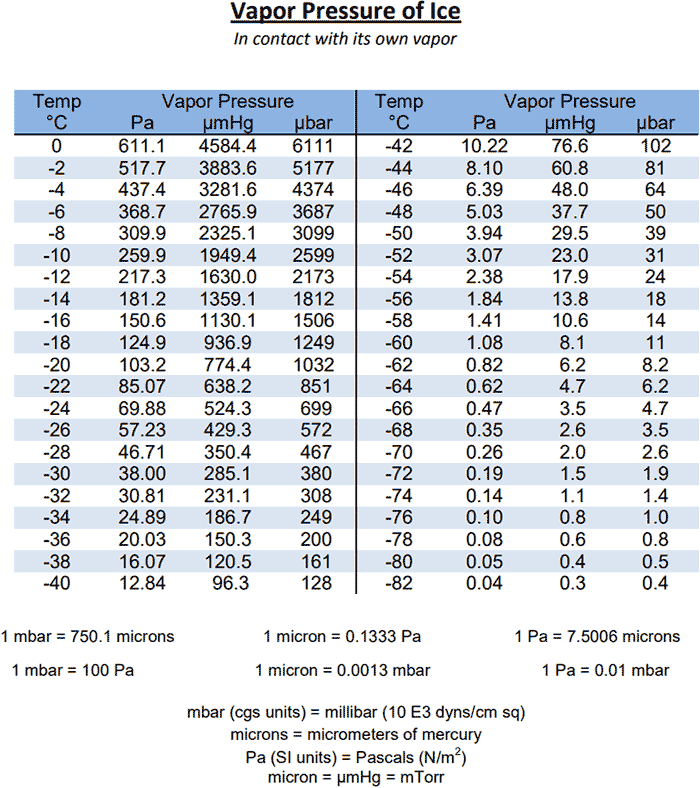
How To Find Vapor Pressure Of Water How can you measure the water
![[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A](https://d3i71xaburhd42.cloudfront.net/471011a1b864be3f78720f4d8ba4f21385acd117/5-Table3-1.png)
[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A
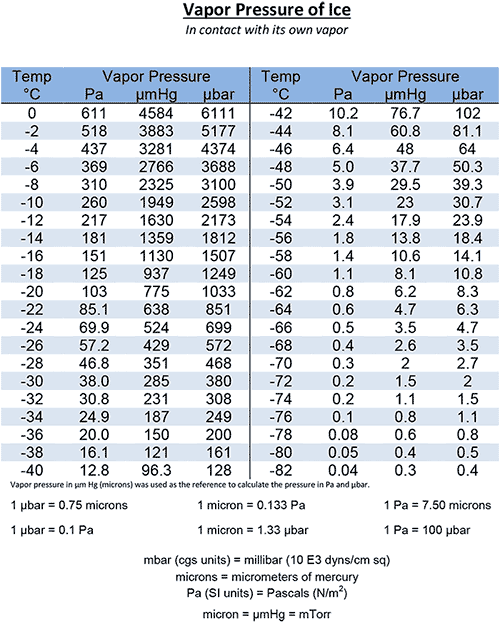
Licenziamento fetta limite water vapor pressure temperature chart
Use One Of The Popular Approximations, E.g., Antoine Formula:
In Other Words, Vapor Pressure Equals Atmospheric Pressure At A Liquid’s Boiling Point.
The Graph Shows How The Saturated Vapor Pressure (Svp) Of Water Varies From 0°C To 100 °C.
Web Vapor Pressure Of Water (Mmhg) At Selected Temperatures (°C) 0 :
Related Post: