Water Vapour Pressure Chart
Water Vapour Pressure Chart - The boiling point of a liquid varies depending upon the surrounding pressure. Plot the data and use your graph to estimate the vapor pressure of water at 25°c and at 75°c. Pw, s = water vapor saturation pressure (pa) t = temperature in kelvin, k = °f + 255.927778. Web vapor pressure of water from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c vp, torr temp, °c vp, torr temp, °c vp, torr 0 4.58 35 41.18 70 233.7 1 4.93 36 44.56 71 243.9 2 5.29 37 47.07 72 254.6 3 5.68 38 49.69 73 265.7 4 6.10 39 52.44 74 277.2 Pw, s = ea + b/t + c lnt +dt. Web the calculator below can be used to calculate the water saturation pressure at given temperatures. Web the boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Using the above chart, the relationship between the pressure and temperature can be seen. Web water vapor pressure chart. Use one of the popular approximations, e.g., antoine formula: Use these data to determine the value of δh vap for water. Pw, s = water vapor saturation pressure (pa) t = temperature in kelvin, k = °f + 255.927778. Therefore, for a given temperature, the associated vapor pressure can. To find the vapor pressure of water: This solution is comprised of chemical a and chemical b. Use one of the popular approximations, e.g., antoine formula: Pressure (degrees c) (mmhg) (degrees c) (mmhg) 0: We also created a water vapor pressure chart ranging from water vapor pressure chart 1°c to 150°c. 2, with the wind and pressure histories shown in figs. Web now according to above formula. The boiling point of a liquid varies depending upon the surrounding pressure. Vapor pressure of water from 0 °c to 100 °c. Every gas in the atmosphere exerts pressure, for example, vapor pressure makes up a fraction of the total atmospheric pressure. What is the vapor pressure of water at 110°c? What is the vapor pressure of water at 343. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. At 393 k the vapor pressure of water is 1489 mmhg; Plot the data and use your graph to estimate the vapor pressure of water at 25°c and at 75°c. Pw, s = ea + b/t + c lnt. Vapor pressure is measured in the standard units of pressure. Every gas in the atmosphere exerts pressure, for example, vapor pressure makes up a fraction of the total atmospheric pressure. To find the vapor pressure of water: Vapor pressure of water at 25°c and 20°c. Selected pressure observations and best track minimum central pressure curve for hurricane. Web the following table gives the vapor pressure of water at various temperatures. In this type of closed system, some molecules of a liquid or solid have enough kinetic energy to escape at the surface and enter the vapor (gas) phase. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of. We did the same thing with the kelvin temperature scale, including a 273k to 400k vapor pressure chart. Pressure (degrees c) (mmhg) (degrees c) (mmhg) 0: Vapor pressure of water at 25°c and 20°c. Web vapour pressure diagrams of water; P antoine = 10 a−b/(c+t) = 10 8.14019−1810.94/(244.485+t) enter t = 80 °c in celsius degrees: Web often, a lookup table or a chart like the one below will be used to find the vapor pressure of a liquid. Vapor pressure is measured in the standard units of pressure. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. The output pressure is given as kpa, bar, atm, psi (lb/in 2 ). Vapour tension / vapour pressure of the liquid = 1.2335 / 0.8 = 1.54 metres. Therefore, for a given temperature, the associated vapor pressure can. A solution's partial pressure is 34.93 mmhg. Web the calculator below can be used to calculate the water saturation pressure at given temperatures. P antoine = 10 a−b/(c+t) = 10 8.14019−1810.94/(244.485+t) enter t = 80. Web now according to above formula. Selected pressure observations and best track minimum central pressure curve for hurricane. Plot the data and use your graph to estimate the vapor pressure of water at 25°c and at 75°c. Graphics shows triple point, critical point and boiling point of water. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0 : At 393 k the vapor pressure of water is 1489 mmhg; The pressure up cancels the pressure down and boiling begins. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. Web the boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Web the vapor pressure of water at 283 k is 9.2 mmhg, at what temperature is the vapor pressure of water 546 mmhg? Saturation (pressure) 11 p, mpa t, °c density, kg/m3 ρ l ρ v enthalpy, kj/kg h lv ∆h entropy, kj/(kg·k) s l s v ∆s volume, cm3/g v v 611.657 pa 0.01 999.79 0.004 855 0.00 2500.9 2500.9 0.000 00 9.1555 9.1555 1.000 21 205 991. Pw, s = ea + b/t + c lnt +dt. Web the following table gives the vapor pressure of water at various temperatures. Web vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. The high surface tension of water (water sticks to itself, so it doesn't want to evaporate) means water has a low vapor pressure. Web water vapor pressure chart. Web the vapor pressure of water at 80 °c will be 47.27 kpa (antoine formula) or 46.19 kpa (simple formula). In the following equation, all of the gases in earth’s atmosphere contribute to the total atmospheric pressure patmosphere. Selected pressure observations and best track minimum central pressure curve for hurricane. Web vapor pressure at saturation. Use these data to determine the value of δh vap for water.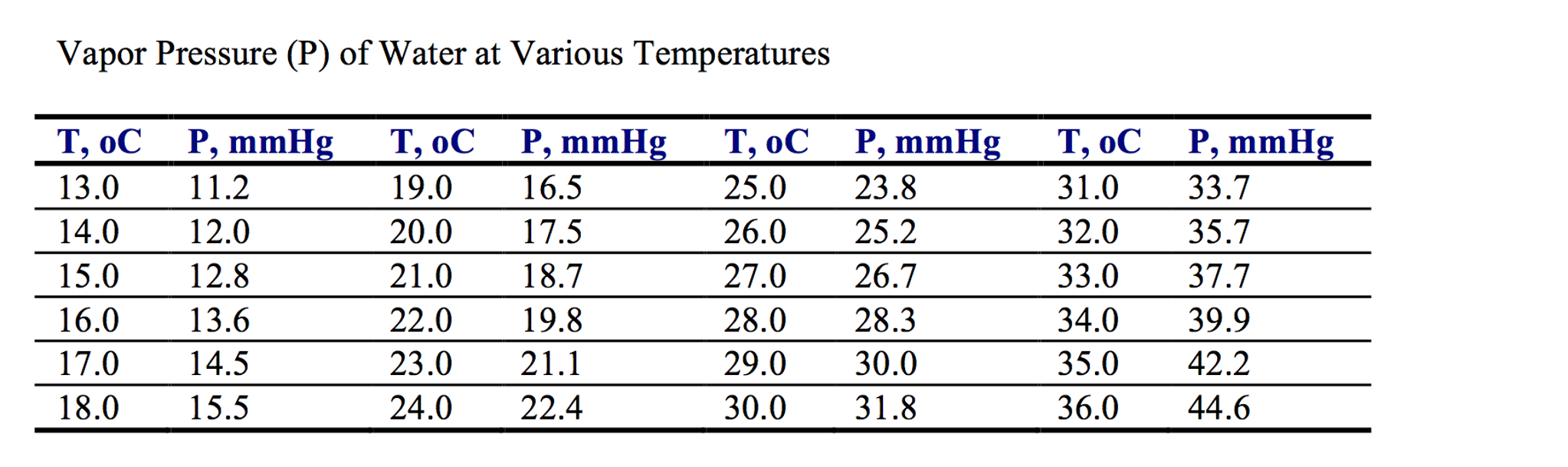
Solved a).Find the vapor pressure of water at 29.6 oC. b)

Conservation physics Fundamental microclimate concepts
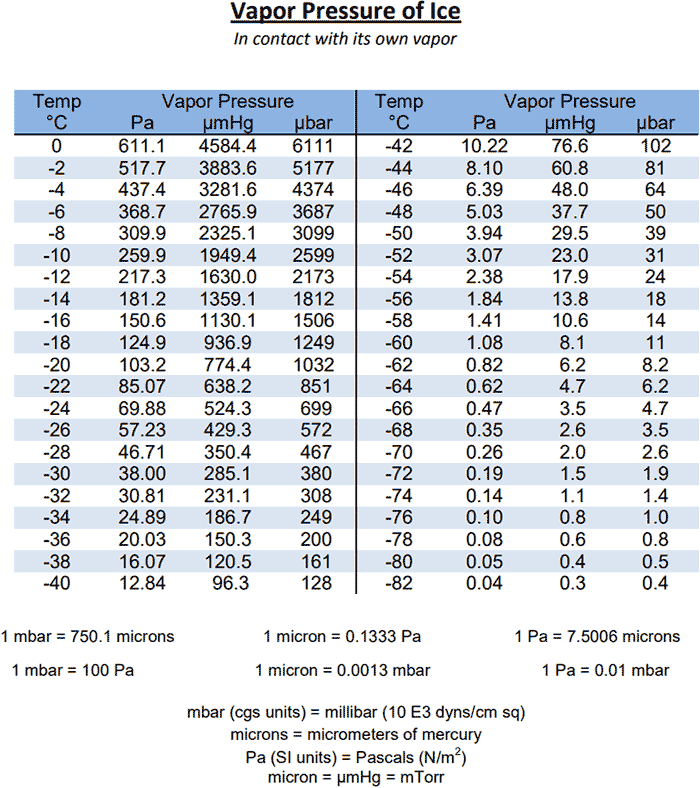
How To Find Vapor Pressure Of Water How can you measure the water
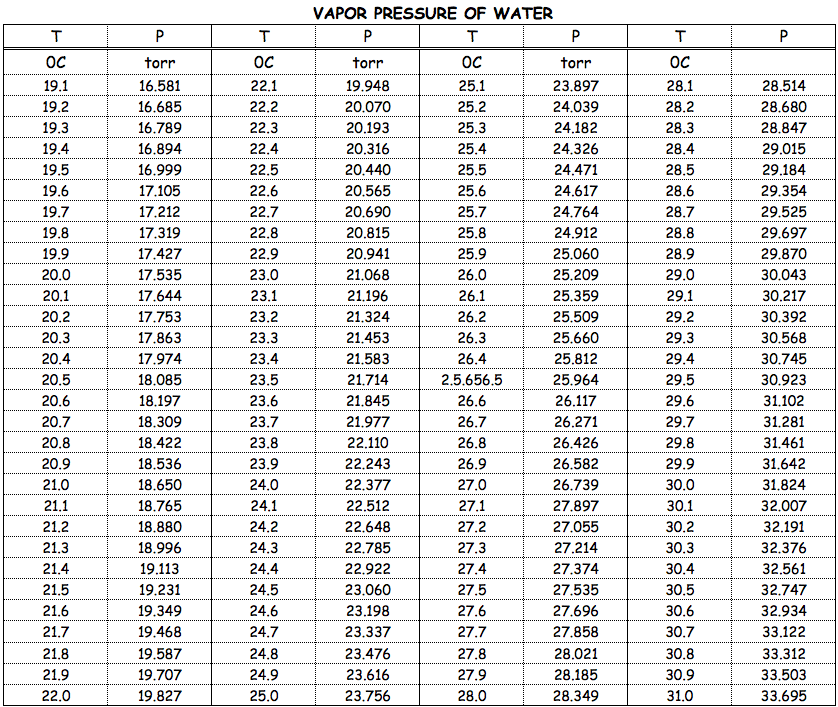
A solution of sodium chloride in water has a vapor pressure of 19.6
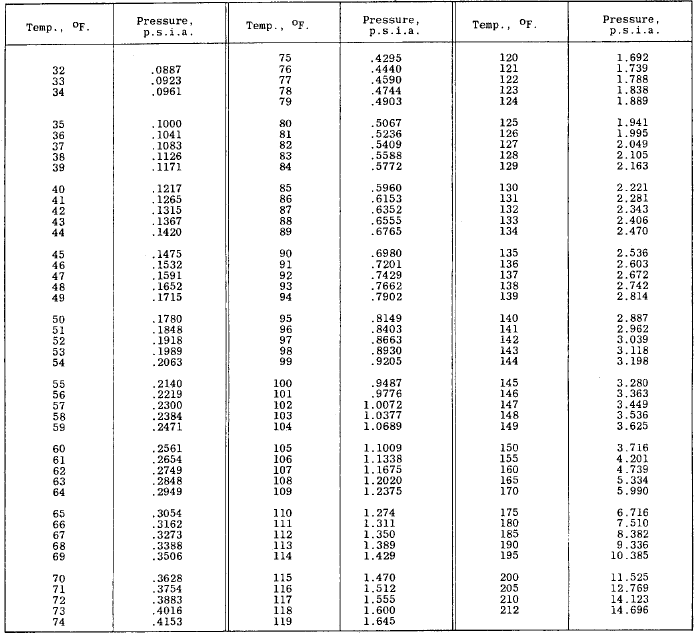
Partial Pressure of Water Vapor in Saturated Air Table Chart
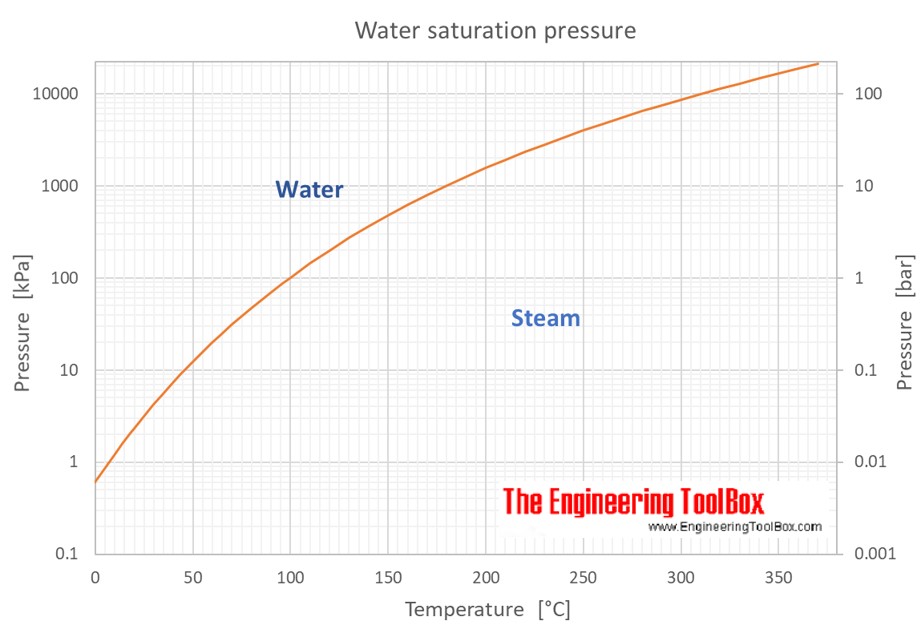
Water Saturation Pressure vs. Temperature
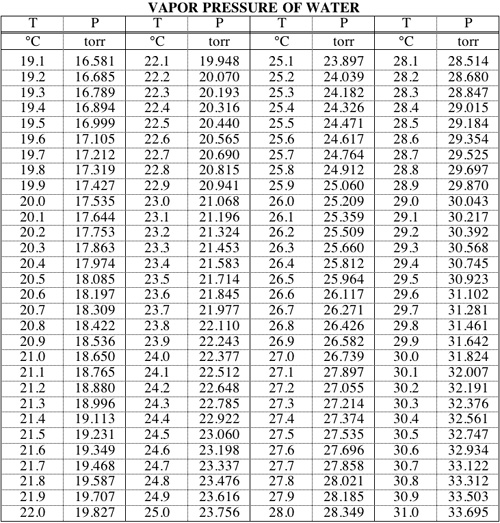
cuvânt înainte hârtie Salut water pressure temperature calculator
Vapour Pressure of Water Vapor Celsius

evaporation Does water need oxygen to evaporate? Physics Stack Exchange

Vapour Pressure Of Water Chart
Vapor Pressure Of Water At 25°C And 20°C.
Plot The Data And Use Your Graph To Estimate The Vapor Pressure Of Water At 25°C And At 75°C.
What Is The Vapor Pressure Of Water At 343 K?
Vapour Tension / Vapour Pressure Of The Liquid = 1.2335 / 0.8 = 1.54 Metres.
Related Post:
