Acid And Base Chart
Acid And Base Chart - What is an acid or a base? For a given acid or base, these equilibria are linked by the water dissociation equilibrium: Ph values of common chemicals. Typical concentrations of these ions in solution can be very small, and they also span a wide range. Whether a liquid is an acid or a base has to do with hydrogen ions (abbreviated with the chemical symbol h + ). Evaluate solution ph and poh of strong acids or bases. This chart is ideal for use in the lab or in the classroom. Make sure you thoroughly understand the following essential concepts: In acidic solution, ph <7; Alkalemia is serum ph > 7.45. Acids, bases, & the ph scale. You’ll see charts that give slightly different ph values for some chemicals. Web chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. Acidemia is serum ph < 7.35. For example,. This chart is ideal for use in the lab or in the classroom. Chart or notebook size available. Properties of acids vs bases. Acids and bases are common substances found in many every day items, from fruit juices and soft drinks to soap. Web the most common strong acids and bases are listed in table 1. Chart or notebook size available. Web chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. We will cover ph, and how to calculate the ph of a solution. Solutions are classified as acidic or basic based. Whether a liquid is an acid or a base has to do with hydrogen ions (abbreviated with the chemical symbol h + ). In water (h 2 o), a small number of. Web give the names and formulas of some strong acids and bases. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. The acid. Explain the ph scale, and convert ph and concentration of hydronium ions. What is an acid or a base? Make sure you thoroughly understand the following essential concepts: An acid is a substance that forms hydrogen ions h + when dissolved in water, and; Acidic solutions have a higher h + concentration than water (greater than 1 × 10 −. A ph scale is a tool for measuring acids and bases. The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. The relative strengths of acids may be quantified by measuring their equilibrium constants in aqueous solutions. Web the most common strong acids and bases are listed in table 1.. Acids and bases are common substances found in many every day items, from fruit juices and soft drinks to soap. In water (h 2 o), a small number of. Typical concentrations of these ions in solution can be very small, and they also span a wide range. They can react with bases to produce salts and water. How to use. Bases have a slippery feel on fingers and taste bitter. How to use a ph indicator. Acids taste sour and create a stinging feeling on the mucous membranes. For a given acid or base, these equilibria are linked by the water dissociation equilibrium: Acidemia is serum ph < 7.35. Evaluate solution ph and poh of strong acids or bases. In aqueous solution, an acid is defined as any species that increases the concentration of h + ( a q) , while a base increases the concentration of oh − ( a q). So what is an acid? In this chapter, we will examine the properties of acids and bases,. In this chapter, we will examine the properties of acids and bases, and learn about the chemical nature of these important compounds. This chart is ideal for use in the lab or in the classroom. Evaluate solution ph and poh of strong acids or bases. In solutions of the same concentration, stronger acids ionize to a greater extent, and so. We will cover ph, and how to calculate the ph of a solution. In water (h 2 o), a small number of. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. The relative strengths of acids may be quantified by measuring their equilibrium constants in aqueous solutions. Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. So what is an acid? The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. Acids taste sour and create a stinging feeling on the mucous membranes. A ph scale is a tool for measuring acids and bases. For students using the foundation edition, essential understanding acids and bases can be classified in terms of hydrogen ions or hydroxide ions, or in terms of electron pairs. An acid is a substance that forms hydrogen ions h + when dissolved in water, and; Web give the names and formulas of some strong acids and bases. Alkalemia is serum ph > 7.45. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Chart or notebook size available. Evaluate solution ph and poh of strong acids or bases.
acids and bases on emaze

Sea Level Diagram Acid Base Tutorial
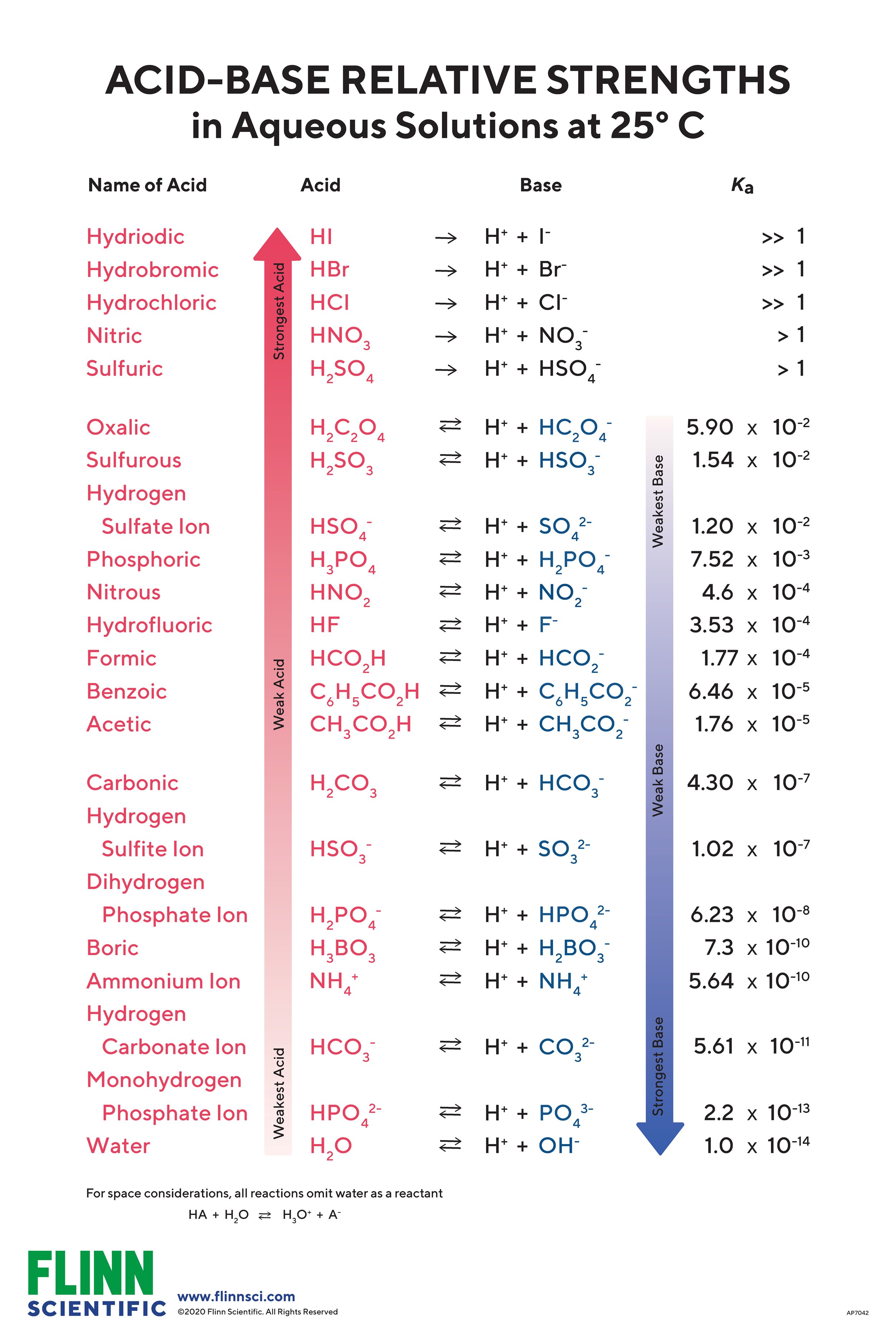
AcidBase Strength Chart
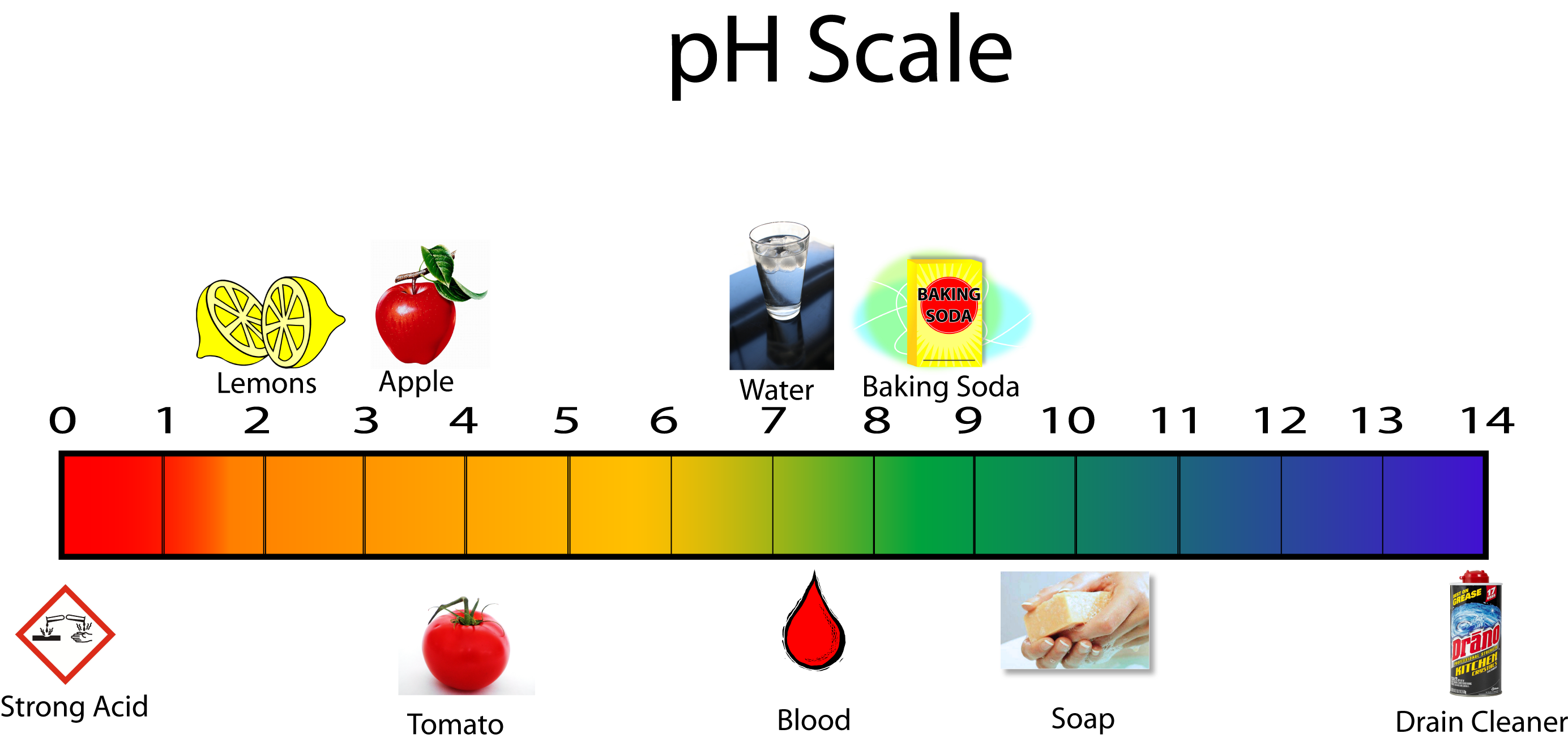
Acids and Bases MCHS Science

Found Out About Chemistry Acidbase indicator charts
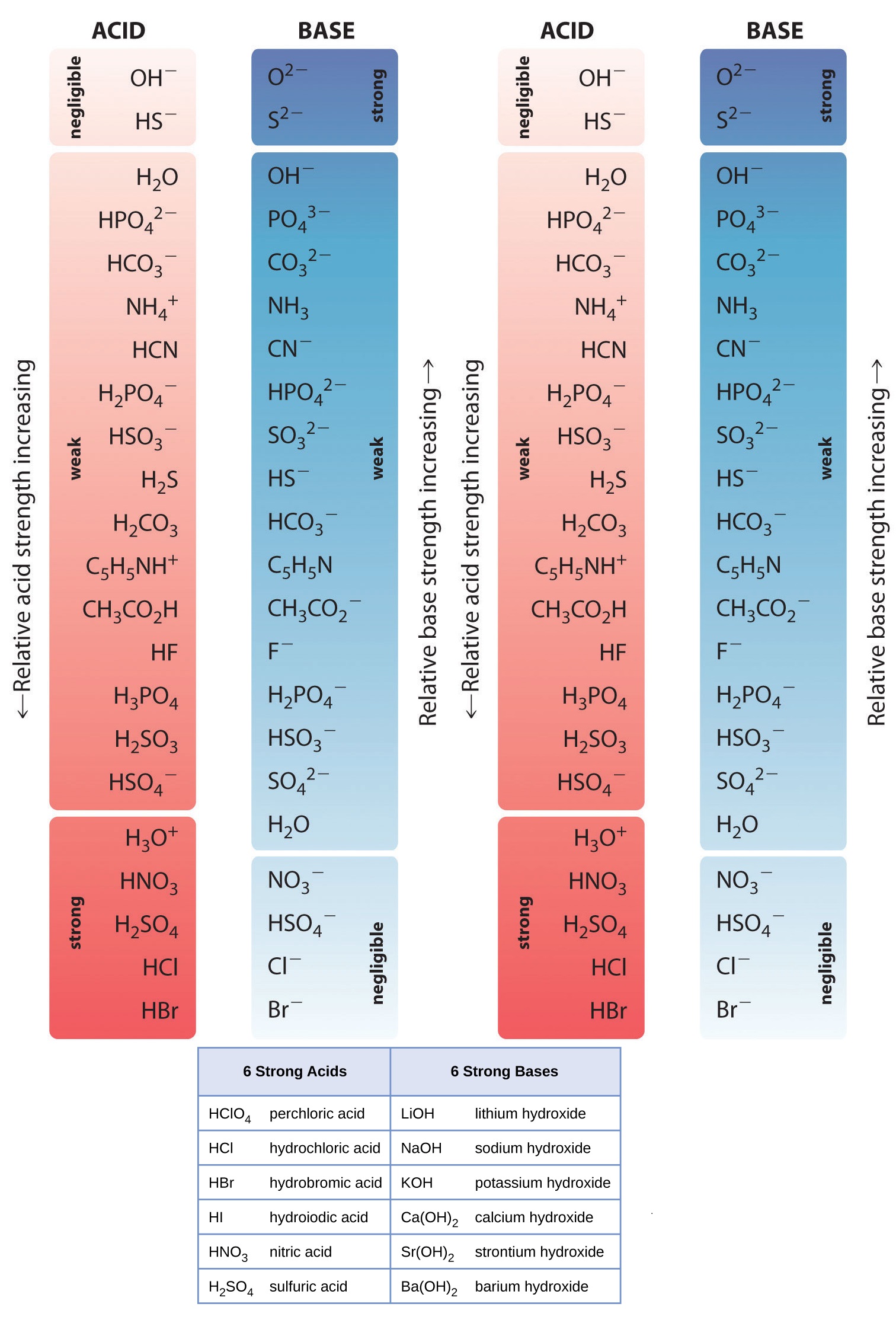
List of Strong Acids & Bases in Order StudyPK
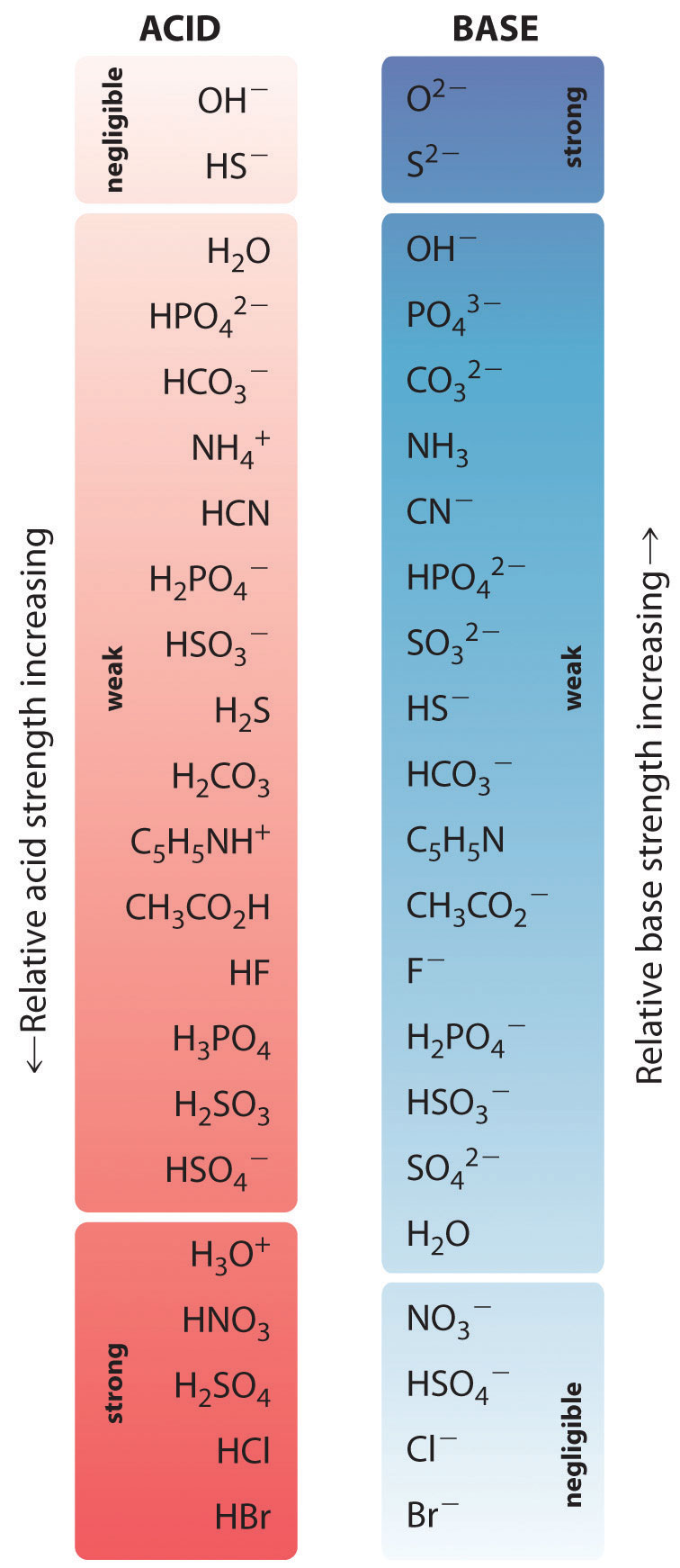
16.5 Strong Acids and Bases Chemistry LibreTexts
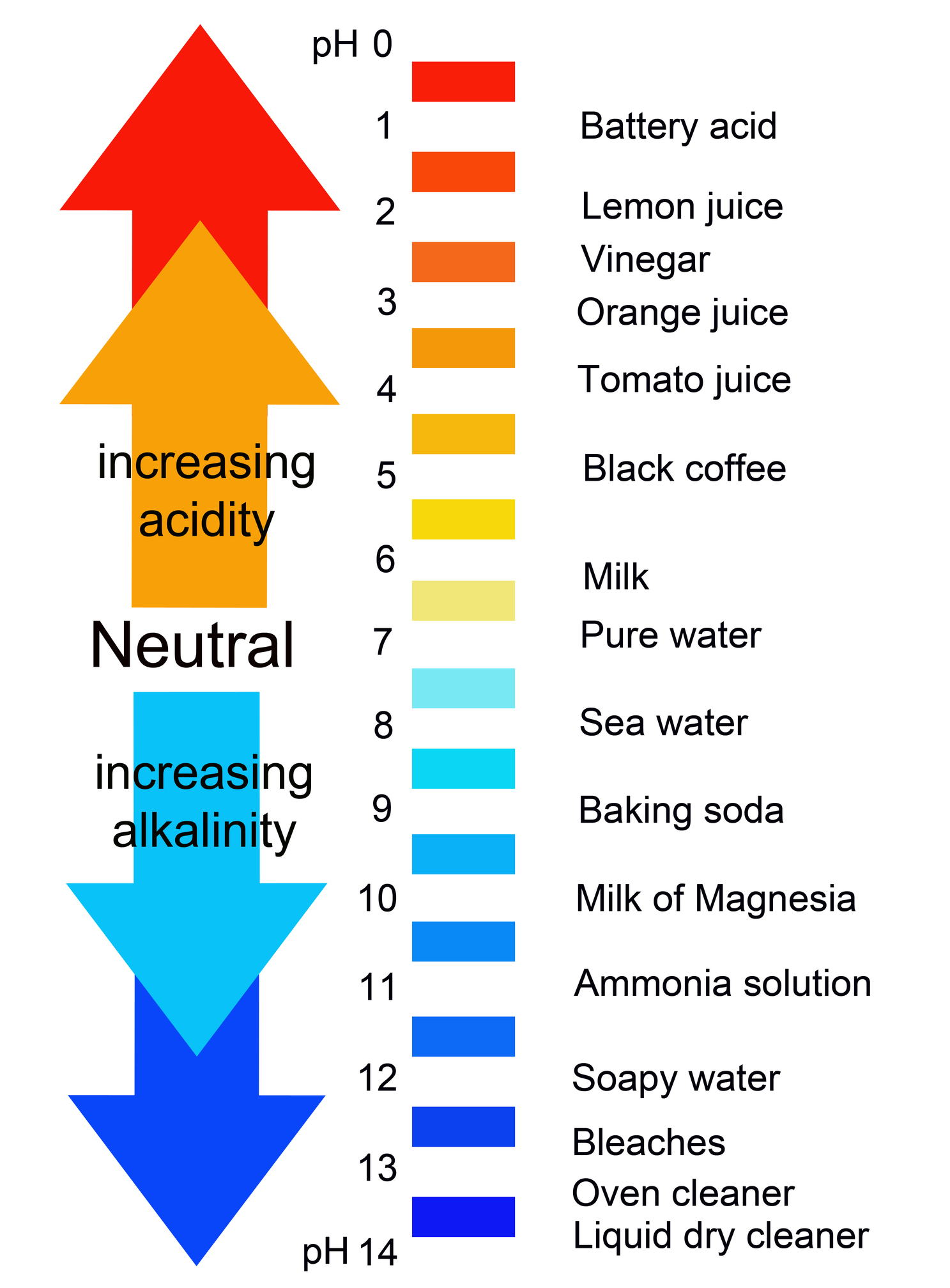
Acids and Bases
Water, Acids, and Bases CK12 Foundation
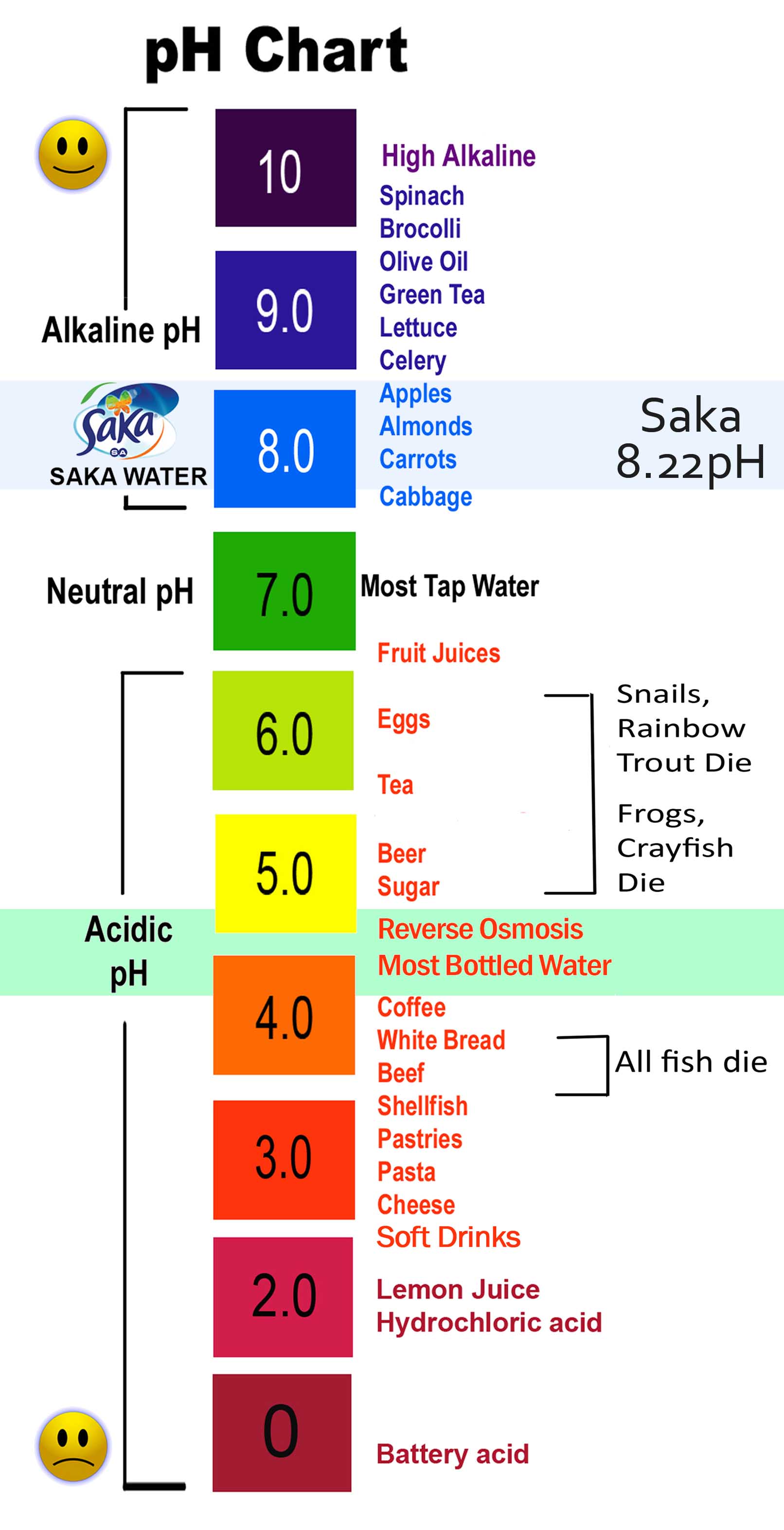
4a. Acids and Bases Antonia's chemistry blog
What Are Acids And Bases?
How To Use A Ph Indicator.
Ph Values Of Common Chemicals.
Web Chemicals With Ph Values From 0 Up To 7 Are Acids, Those With A Ph Value Of 7 Are Neutral, And Those With Ph Values Greater Than 7 Up To 14 Are Bases.
Related Post:
